PTC Therapeutics Reports FDA Acceptance and Priority Review of Upstaza™ BLA
PTC Therapeutics, Inc. revealed today that the FDA has officially received the Biologics License Application for Upstaza™ (eladocagene exuparvovec), a gene therapy aimed at treating AADC deficiency. The submission has been given Priority Review status, with a projected regulatory decision date set for November 13, 2024.
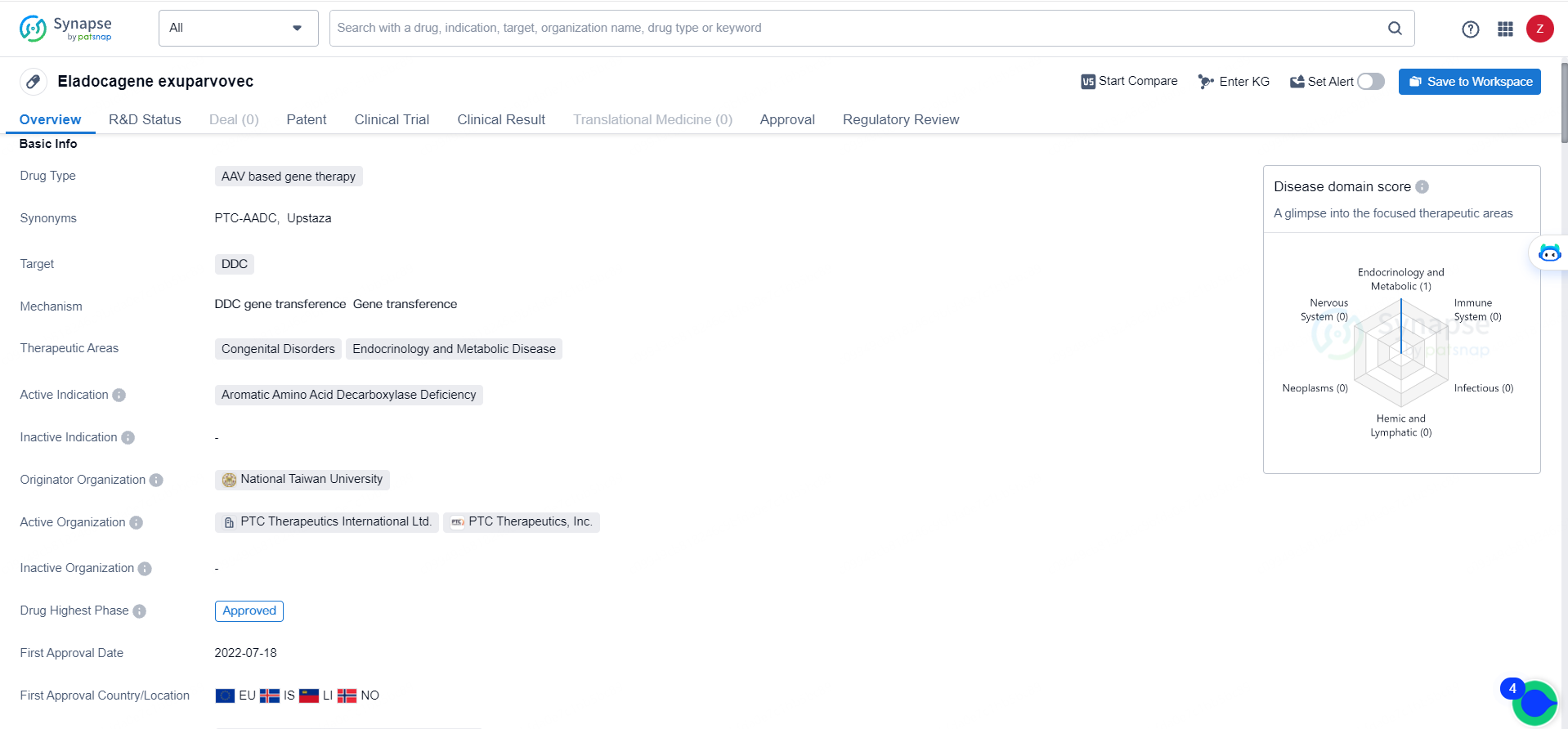
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Matthew B. Klein, M.D., Chief Executive Officer of PTC Therapeutics, expressed enthusiasm about advancing towards providing an approved treatment for patients in the U.S. who suffer from AADC deficiency. "The cumulative data thus far supports the significant transformative potential of Upstaza, a pioneering gene therapy that is administered directly to the brain," he remarked.
Designed as a one-time gene replacement therapy, Upstaza is meant for patients 18 months and older diagnosed clinically, molecularly, and genetically with severe aromatic L–amino acid decarboxylase deficiency. This treatment employs a recombinant adeno-associated virus serotype 2 (AAV2) vector containing the human DDC gene to correct the genetic defect. By delivering the functional DDC gene directly into the putamen, Upstaza aims to boost AADC enzyme levels and restore dopamine synthesis.
Clinical trials and compassionate use programs have consistently shown Upstaza’s efficacy and safety profile since the first patient was treated in 2010. These trials have highlighted major neurological improvements, with initial insomnia, irritability, and dyskinesia being the most frequent side effects.
Upstaza is delivered via a stereotactic surgical method, a minimally invasive procedure frequently used to address various pediatric and adult neurological disorders. This procedure, specific to specialized neurosurgery centers, is conducted by trained neurosurgeons.
Eladocagene exuparvovec, which targets the DDC gene, is indicated for treating Aromatic Amino Acid Decarboxylase Deficiency within the therapeutic domains of endocrinology and metabolic diseases. The drug received its initial approval in the European Union, Iceland, Liechtenstein, and Norway in July 2022, following an expedited review by health authorities.
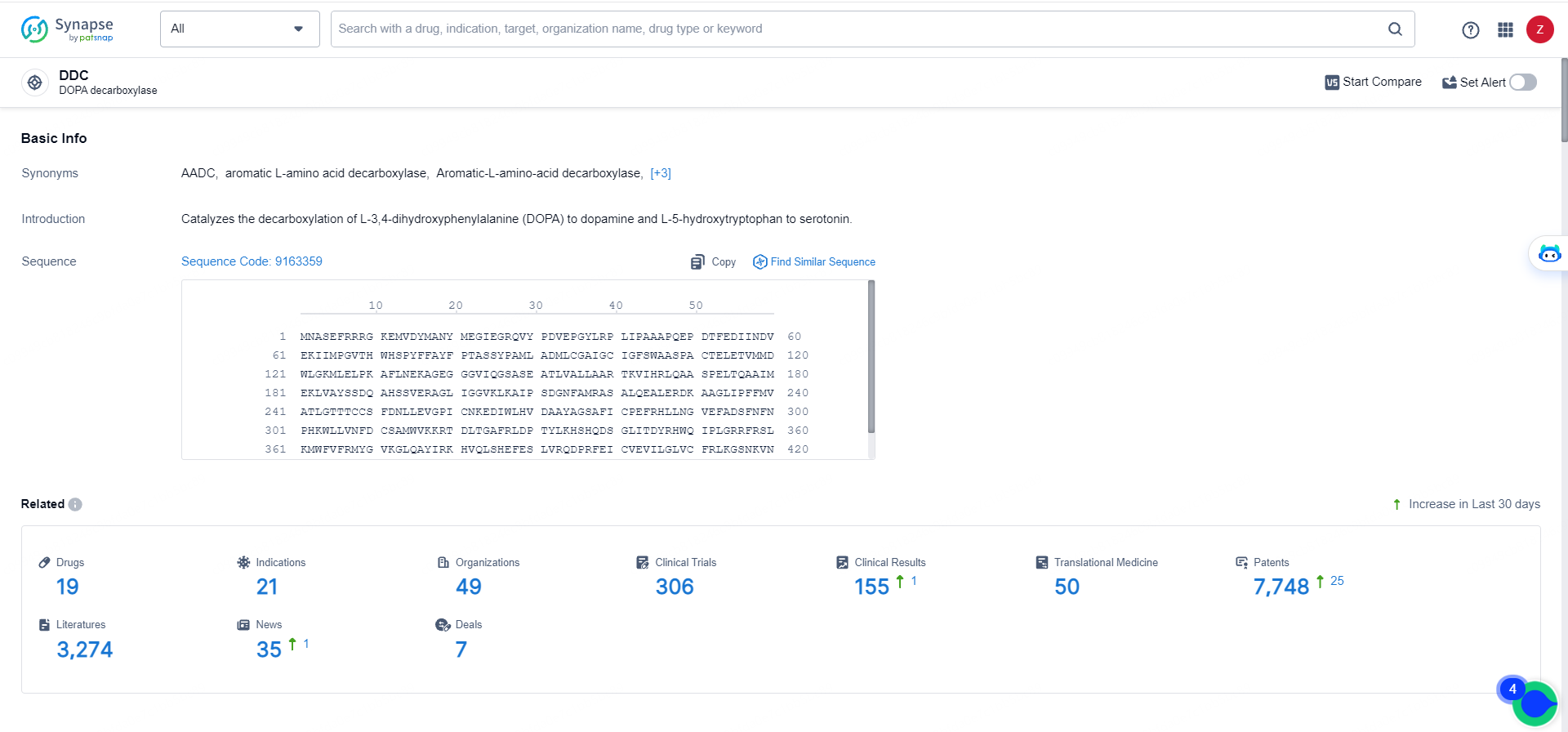
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of May 16, 2024, there are 19 investigational drugs for the DDC target, including 21 indications,49 R&D institutions involved, with related clinical trials reaching 306, and as many as 7748 patents.
The approval of Eladocagene exuparvovec represents a significant achievement in the pharmaceutical industry, offering a promising new treatment option for patients with Aromatic Amino Acid Decarboxylase Deficiency. As the drug continues to be evaluated and potentially approved in other regions, it has the potential to make a meaningful impact on the lives of individuals affected by this rare genetic disorders.