Pilocarpine Hydrochloride Unveiled: A Detailed Overview of its Revolutionary R&D Breakthroughs
Pilocarpine Hydrochloride's R&D Progress
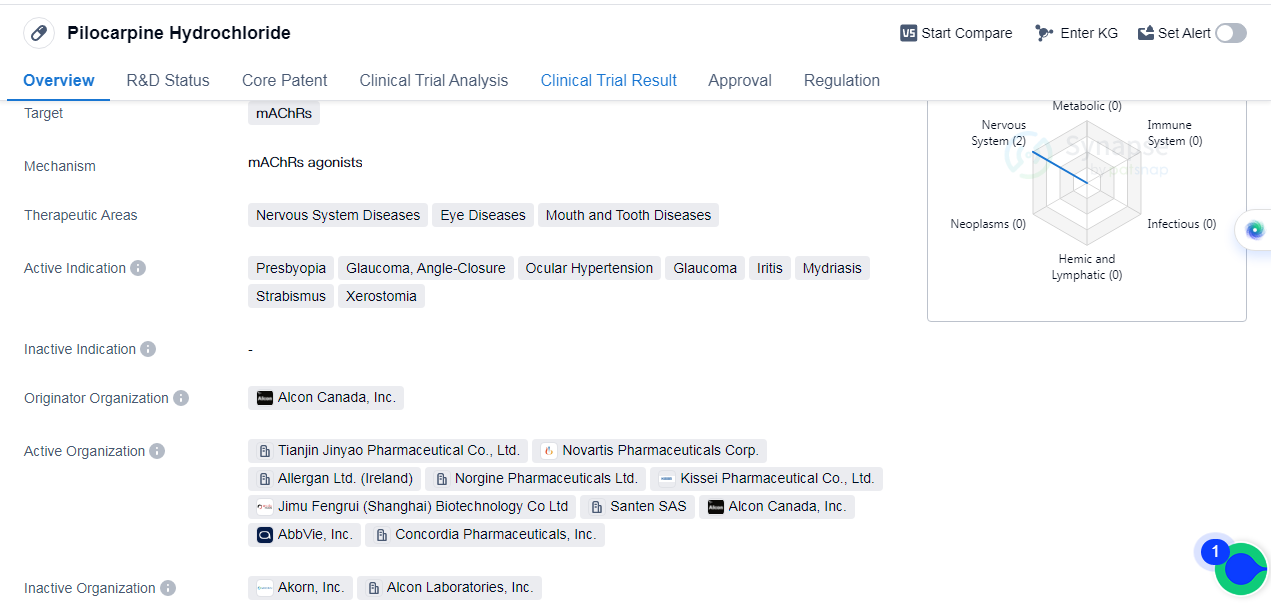
Pilocarpine Hydrochloride is a small molecule drug that primarily targets muscarinic acetylcholine receptors (mAChRs). It is used in the treatment of various nervous system diseases, eye diseases, and mouth and tooth diseases. The drug has been indicated for treating presbyopia, glaucoma (both angle-closure and ocular hypertension), iritis, mydriasis, strabismus, and xerostomia.
The originator organization of Pilocarpine Hydrochloride is Alcon Canada, Inc. The drug has received approval for use in multiple countries, including Canada, where it was first approved in December 1951. Pilocarpine Hydrochloride is classified as an orphan drug, indicating that it is used to treat rare diseases or conditions.
As a small molecule drug, Pilocarpine Hydrochloride is designed to interact with specific receptors in the body, in this case, mAChRs. By targeting these receptors, the drug aims to alleviate symptoms associated with various diseases affecting the nervous system, eyes, and mouth.
The therapeutic areas of Pilocarpine Hydrochloride encompass a range of conditions, including presbyopia, which is the age-related loss of near vision, and glaucoma, a group of eye diseases characterized by damage to the optic nerve. The drug is also used in the treatment of angle-closure glaucoma, ocular hypertension, iritis (inflammation of the iris), mydriasis (dilation of the pupil), strabismus (misalignment of the eyes), and xerostomia (dry mouth).
Pilocarpine Hydrochloride has achieved the highest phase of approval in the global markets. This indicates that the drug has undergone extensive clinical trials and has been deemed safe and effective for use in patients. The drug's long history, with its first approval dating back to 1951, further demonstrates its established position in the pharmaceutical industry.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Pilocarpine Hydrochloride: mAChRs agonists
mAChRs agonists refers to agonists that specifically target and activate muscarinic acetylcholine receptors (mAChRs). Muscarinic acetylcholine receptors are a type of G protein-coupled receptor found in various tissues and organs throughout the body, including the central nervous system, cardiovascular system, and smooth muscle.
When mAChRs agonists bind to and activate these receptors, they mimic the effects of acetylcholine, the natural neurotransmitter that normally binds to mAChRs. This activation can lead to a wide range of physiological responses depending on the specific mAChR subtype and the location of the receptor.
In the context of biomedicine, mAChRs agonists have been studied and utilized for their therapeutic potential in various conditions. For example, they can be used to treat certain neurological disorders such as Alzheimer's disease, where mAChR function is impaired. By activating mAChRs, these agonists can help improve cognitive function and memory.
Additionally, mAChRs agonists can be used to stimulate smooth muscle contraction in the gastrointestinal tract, urinary bladder, and other organs. This can be beneficial in cases of urinary retention or gastrointestinal motility disorders.
Overall, mAChRs agonists play a crucial role in modulating various physiological processes and have therapeutic applications in the treatment of several medical conditions.
Drug Target R&D Trends for Pilocarpine Hydrochloride
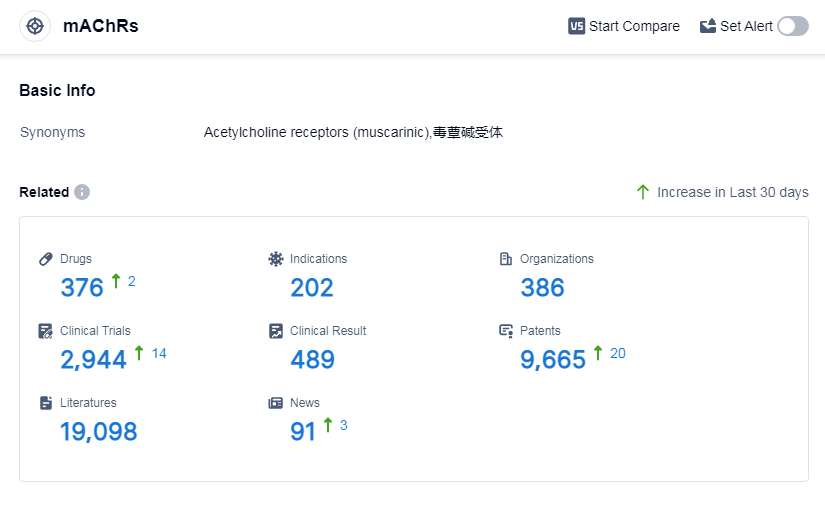
According to Patsnap Synapse, as of 7 Sep 2023, there are a total of 376 mAChRs drugs worldwide, from 386 organizations, covering 202 indications, and conducting 2944 clinical trials.
The analysis of target mAChRs reveals a competitive landscape with multiple companies actively developing drugs. Pfizer Inc., C.H. Boehringer Sohn AG & Co. KG, Santen Pharmaceutical Co., Ltd., AstraZeneca PLC, and Astellas Pharma, Inc. are the companies growing fastest under this target.
The indications with the highest number of approved drugs include pulmonary disease, chronic obstructive, urinary bladder, overactive, spasm, and duodenal ulcer. These indications have a significant number of approved drugs, indicating a focus on addressing unmet medical needs.
Small molecule drugs dominate the development landscape for mAChRs, with biosimilars such as monoclonal antibodies also present. This suggests intense competition and potential for innovation in this field.
China, Japan, and the United States are the leading countries in terms of drug development for mAChRs, with China showing significant progress. The European Union, Canada, and other countries also contribute to the development of drugs targeting mAChRs.
Overall, the target mAChRs present a competitive landscape with a focus on specific indications and drug types. The future development of this target will depend on the success of ongoing clinical trials, regulatory approvals, and the emergence of innovative therapies.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Pilocarpine Hydrochloride is a small molecule drug developed by Alcon Canada, Inc. It targets mAChRs and is used in the treatment of various nervous system diseases, eye diseases, and mouth and tooth diseases. The drug has received approval in multiple countries, including Canada, and is classified as an orphan drug. Its therapeutic indications include presbyopia, glaucoma, iritis, mydriasis, strabismus, and xerostomia.