Positive Phase 1a Safety, Tolerability, and PK/PD Results for Tectonic Therapeutic's TX45
Tectonic Therapeutic, Inc. (NASDAQ: TECX) (Tectonic), a clinical-stage biotechnology firm dedicated to discovering and developing therapeutic proteins and antibodies aimed at modulating G-protein coupled receptors (GPCRs), has revealed positive topline results from its Phase 1a trial of TX45, a long-acting relaxin that has the potential to be best-in-class. TX45 is currently under development for treating Group 2 PH-HFpEF.
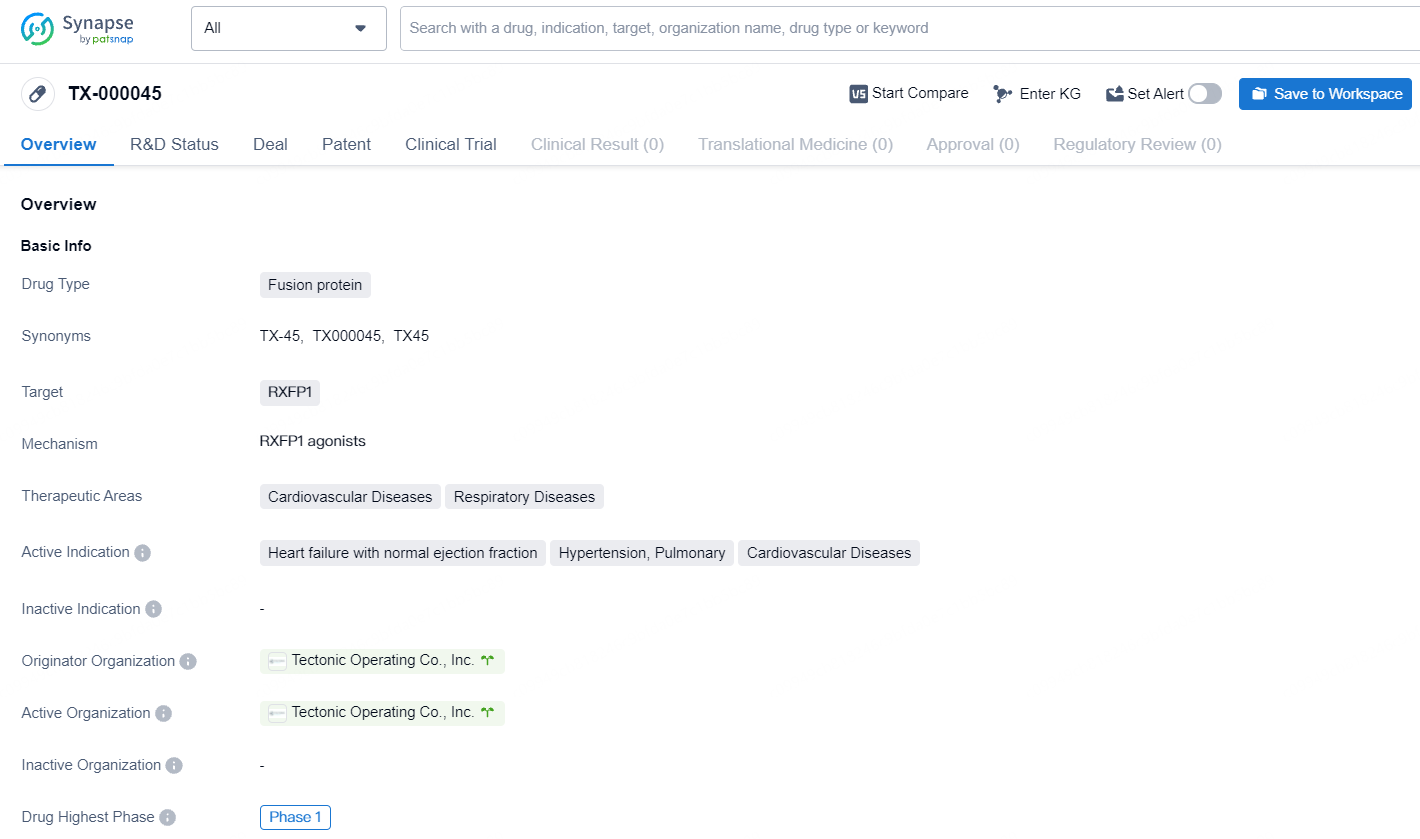
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"These primary Phase 1a findings for TX45 corroborate the initial data previously shared at lower doses, and we anticipate presenting the complete trial results at the AHA Scientific Sessions later this year," stated Alise Reicin, M.D., President and Chief Executive Officer of Tectonic. "Additionally, the Phase 1a data indicate the successful conversion of a sturdy preclinical model into clinical settings, enabling us to select dosages for our global Phase 2 randomized, 6-month clinical trial assessing the impact of TX45 on PH-HFpEF patients, particularly those with combined pre- and post-capillary PH."
The Phase 1a clinical trial is a single ascending dose study conducted in healthy volunteers to evaluate the safety, tolerability, and the pharmacokinetic (PK) and pharmacodynamic (PD) profile of TX45, capitalizing on relaxin's known effect of increasing renal plasma flow. The study investigated TX45 doses of 0.3, 1, and 3 mg/kg delivered intravenously (IV), as well as 150, 300, and 600 mg administered subcutaneously (SC). Findings indicated minimal adverse events and no immune-mediated clearance with TX45. A robust PK/PD relationship was determined by measuring changes in renal plasma flow from baseline at multiple time points post-dosing, aiding in the identification of Phase 2 doses and dosing schedules.
In a preclinical model of chronic pulmonary hypertension, trough levels linked to maximum activity also showed near-peak enhancements in renal plasma flow. The PK/PD model from the Phase 1a clinical data closely matched, with adjusted species potency differences, the PK/PD relationship observed in preclinical studies. Based on these models, Tectonic selected dose regimens for the Phase 2 proof of concept clinical trial in PH-HFpEF patients. In the Phase 2 trial, patients will be randomized to receive either 300 mg SC (2ml injection) once a month of TX45, 300 mg SC every other week, or a placebo.
There are approximately 6 million heart failure patients in the U.S., with HFpEF constituting up to around 50% of these cases. The combined Group 2 PH population with HFpEF is conservatively estimated to exceed 600,000, and there are currently no commercialized treatments available.
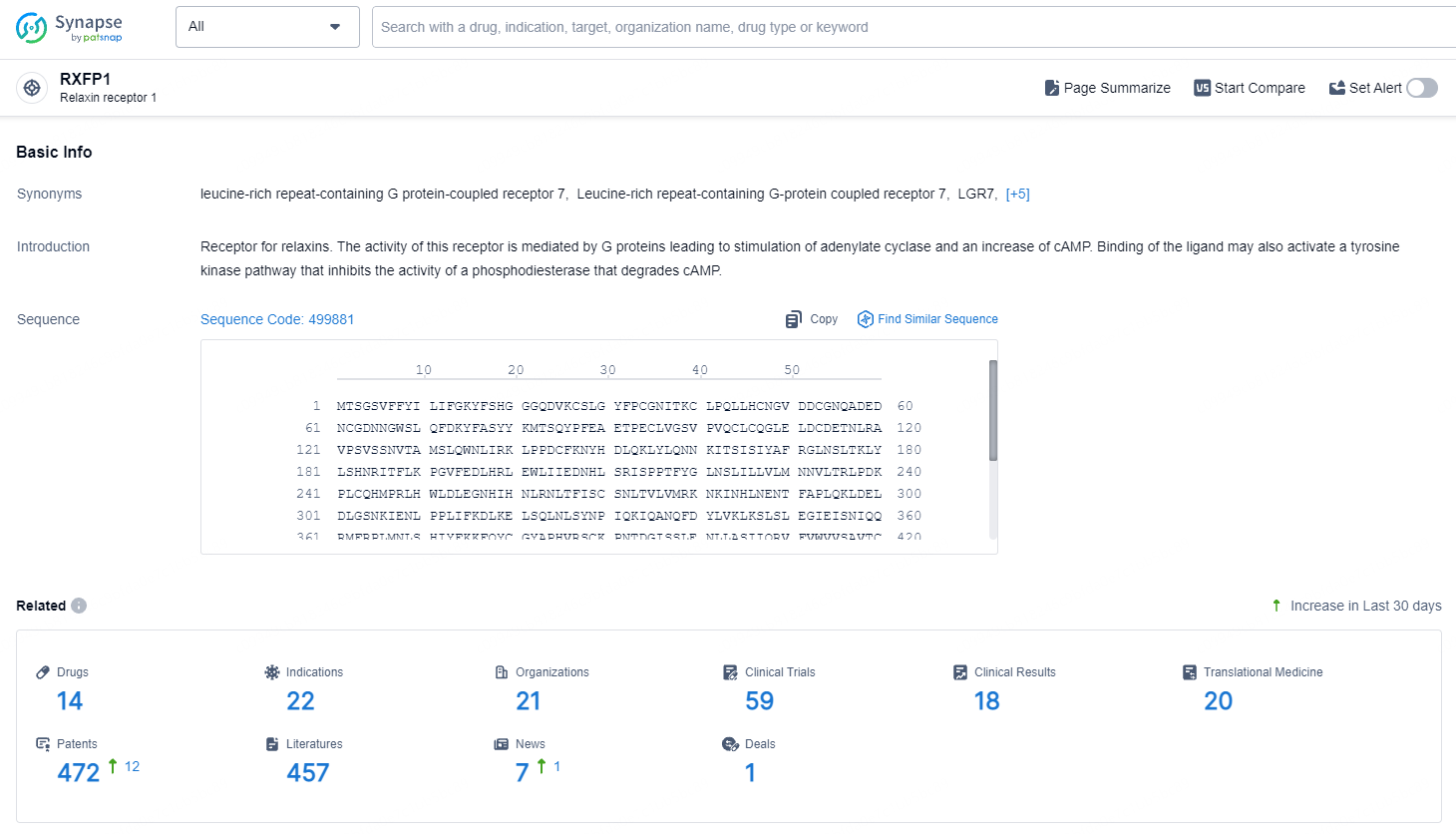
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 23, 2024, there are 14 investigational drusg for the RXFP1 targets, including 22 indications, 21 R&D institutions involved, with related clinical trials reaching 59, and as many as 472 patents.
The drug TX-000045 is a fusion protein that targets RXFP1 and is being developed for the therapeutic areas of cardiovascular diseases and respiratory diseases. The active indications for this drug include heart failure with normal ejection fraction, hypertension, and pulmonary cardiovascular diseases. The originator organization for this drug is Tectonic Operating Co., Inc. As of the latest available information, the drug has reached the highest phase of development, which is Phase 1 on a global scale.