Prazepam Unveiled: A Detailed Overview of its Revolutionary R&D Breakthroughs
Prazepam's R&D Progress
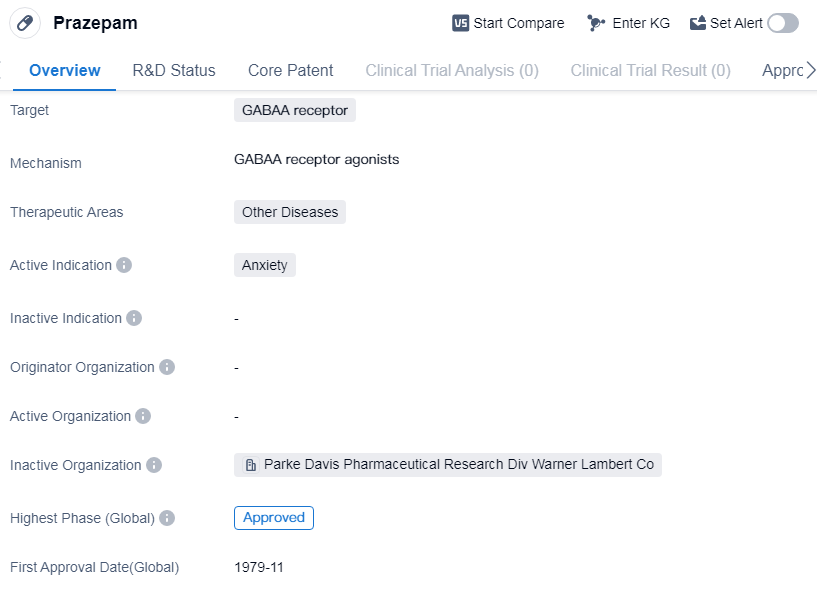
Prazepam is a small molecule drug that targets the GABAA receptor. It falls under the therapeutic area of other diseases and is primarily used for the treatment of anxiety. The drug has received approval for use in various countries globally, with its highest phase being approved.
Prazepam was first approved in the United States in November 1979, making it a well-established medication in the pharmaceutical market. As a small molecule drug, it is designed to interact with the GABAA receptor, which is involved in the regulation of anxiety and other neurological processes.
The drug's therapeutic area is categorized as other diseases, indicating that it may have applications beyond anxiety treatment. However, the specific indications for which Prazepam is approved are not provided in the given information.
Being approved in multiple countries suggests that Prazepam has undergone rigorous testing and evaluation to ensure its safety and efficacy. This global approval also indicates that the drug has met the necessary regulatory requirements in different regions.
The fact that Prazepam has reached the highest phase of approval suggests that it has successfully completed clinical trials and demonstrated its effectiveness in treating anxiety. This milestone is significant as it signifies that the drug has met the necessary standards for safety, efficacy, and quality.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Prazepam: GABAA receptor agonists
GABAA receptor agonists are a type of drugs that bind to and activate the GABAA receptors in the brain. GABAA receptors are a class of receptors that respond to the neurotransmitter gamma-aminobutyric acid (GABA), which is the main inhibitory neurotransmitter in the central nervous system. When GABAA receptors are activated by agonists, they enhance the inhibitory effects of GABA, leading to a decrease in neuronal excitability and a calming or sedative effect.
From a biomedical perspective, GABAA receptor agonists are commonly used in the treatment of various conditions such as anxiety disorders, insomnia, epilepsy, and muscle spasms. By enhancing GABA-mediated inhibition, these drugs help to reduce excessive neuronal activity and promote relaxation and sleep. However, it's important to note that GABAA receptor agonists can also have side effects such as drowsiness, impaired coordination, and dependence with prolonged use. Therefore, their use should be carefully monitored and prescribed by healthcare professionals.
Drug Target R&D Trends for Prazepam
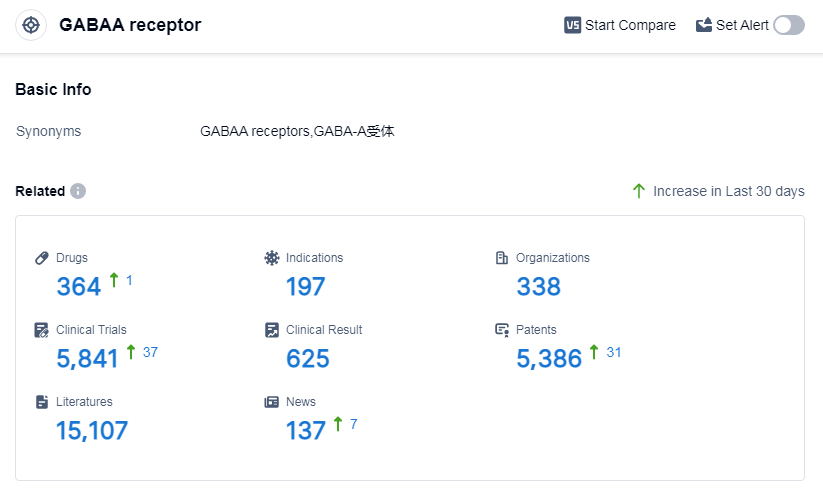
According to Patsnap Synapse, as of 11 Sep 2023, there are a total of 364 GABAA receptor drugs worldwide, from 338 organizations, covering 197 indications, and conducting 5841 clinical trials.
The analysis of the target GABAA receptor reveals a competitive landscape with multiple companies actively involved in research and development. Pfizer Inc., Sumitomo Chemical Co., Ltd., Teva Pharmaceutical Industries Ltd., Jiangsu Nhwa Pharmaceutical Co., Ltd., and Mitsubishi Chemical Group Corp. are some of the companies with a high number of drugs in the approved phase. Sleep initiation and maintenance disorders, anesthesia, and anxiety are the most common indications for approved drugs. Small molecule drugs are progressing rapidly under the current target, with a significant number of drugs in the approved and inactive phases. China is showing progress in the development of drugs targeting the GABAA receptor. Overall, the target GABAA receptor presents opportunities for further research and development in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Prazepam is a small molecule drug that targets the GABAA receptor and is primarily used for the treatment of anxiety. It has received approval in multiple countries, with its highest phase being approved. The drug's first approval was in the United States in November 1979, indicating its long-standing presence in the pharmaceutical market.