Promising Phase 1B Trial Results of VRON-0200 in Chronic Hepatitis B Premiered at APASL 2024
Virion Therapeutics, LLC, an enterprise in the realm of clinical-stage biotech, is actively progressing in the creation of innovative immunotherapies centered around T cell activation. The company has recently unveiled encouraging initial safety outcomes deriving from human trials using its groundbreaking checkpoint modulator, VRON-0200, formulated to target a functional cure for HBV. These findings were delivered by Prof. Grace Wong, M.D., representing the Chinese University of Hong Kong, through a late-breaking oral presentation during the 33rd iteration of the APASL Annual Conference held in Kyoto, Japan.
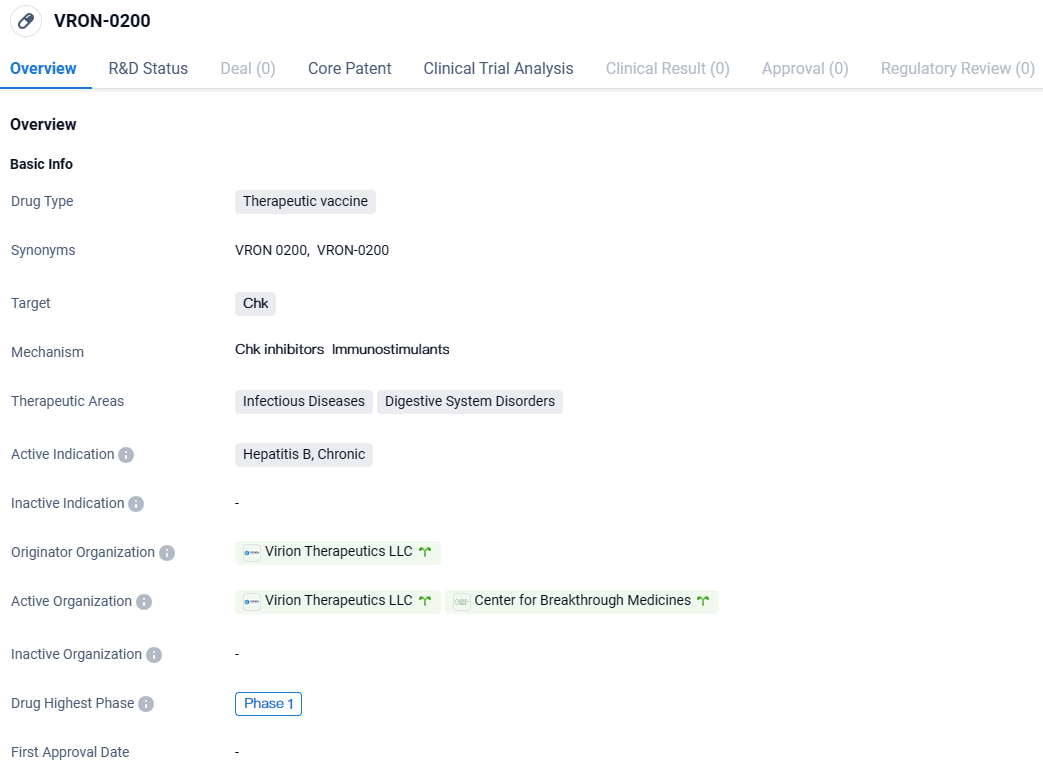
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Dr. Wong stated, "Considering the substantial global figure of nearly 300 million individuals struggling with chronic hepatitis B, there exists a dire need for a treatment option that is both potent in effect and possesses a safe profile. Initial clinical evidence indicates that VRON-0200 has a favorable safety profile and is well-received when administered as a single dose via intramuscular injection in the arm. I am eagerly anticipating the release of additional data regarding safety and other clinical outcomes in the near future."
Dr. Sue Currie, COO of Virion and a co-author of the study, remarked, "The data collected on VRON-0200 is pioneering in its category, being the first to explore a checkpoint modifier T cell vaccine. It's a significant milestone toward the ultimate goal of Virion, which is to develop an efficacious and well-tolerated, as well as easily administered, interferon-sparing therapeutic approach to treating HBV-infected individuals around the globe."
Dr. Chirinjeev Kathuria, co-founder and Executive Chairman of Ocean Biomedical, added, "Our team extends hearty congratulations to Virion, our collaborative partner, on the unveiling of this unprecedented clinical data. These early findings demonstrate the potential for their checkpoint modifier-based immunotherapy to be administered effectively and without undue risk to patients enduring chronic infection with HBV."
VRON-0200 is an innovative immunotherapeutic approach to tackling chronic hepatitis B. It is delivered by injection into the muscle and is crafted with the objective of achieving a functional cure for the condition. The problem lies in the virus's ability to activate HBV-specific CD8+ T cells. However, for individuals who are unable to overcome the initial infection, these T cells may become overtaxed and cease to proliferate or effectively manage the virus. Preclinical studies suggest that by utilizing checkpoint modification, VRON-0200 could potentiate, diversify, and bolster T cell responses, potentially activating cells that chronic HBV infection typically does not, leading to enhanced viral containment.
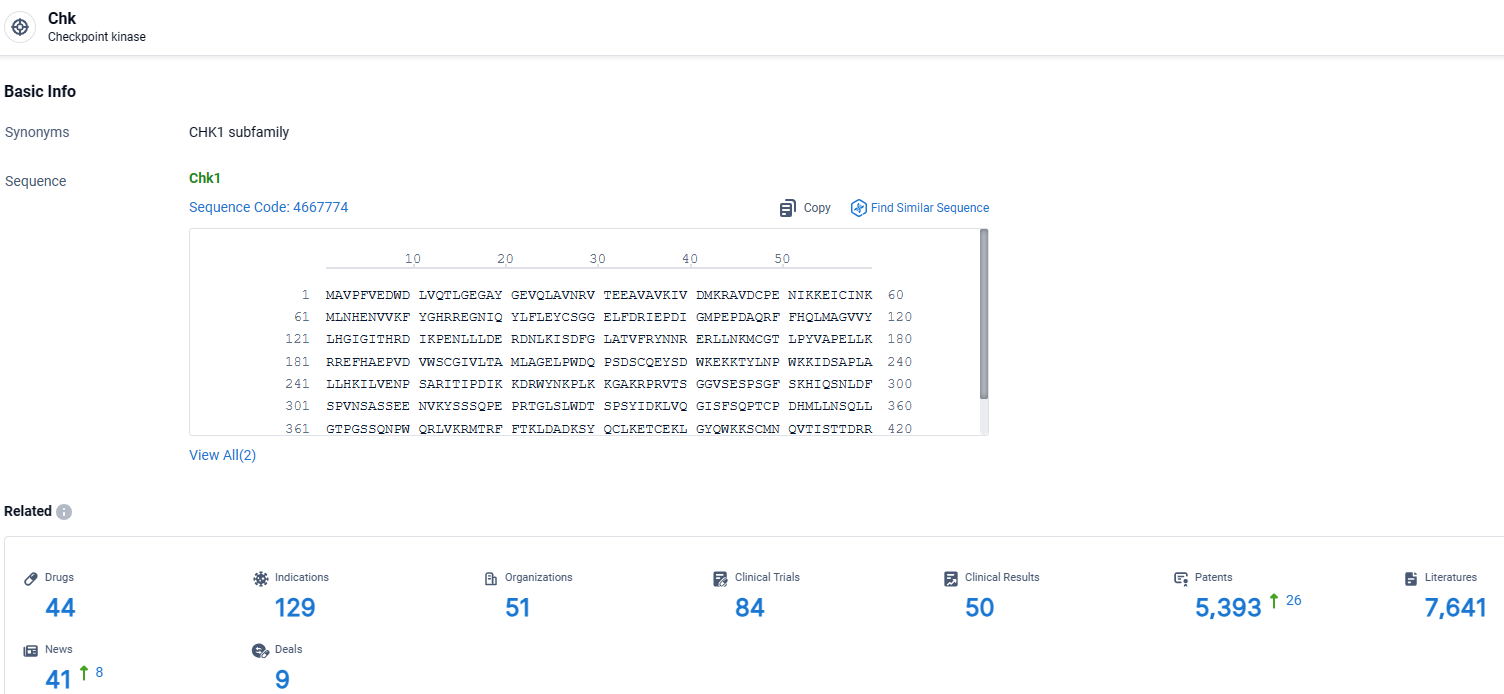
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of April 2, 2024, there are 44 investigational drugs for the Chk target, including 129 indications, 51 R&D institutions involved, with related clinical trials reaching 84, and as many as 5393 patents.
VRON-0200 targets the Chk protein and is primarily intended for the treatment of chronic Hepatitis B. The drug is currently in Phase 1 of clinical development, where its safety, tolerability, and initial efficacy are being evaluated. The potential therapeutic areas of infectious diseases and digestive system disorders suggest broader applications for VRON-0200 beyond Hepatitis B.