Promising Phase I Results for Genentech's Oral GLP-1 Receptor Agonist CT-996 in Treating Obesity
Genentech, part of the Roche Group, revealed favorable topline outcomes from two segments of an ongoing multi-part Phase I clinical trial assessing CT-996. This investigational, once-daily, oral small molecule GLP-1 receptor agonist is being developed to treat type 2 diabetes and obesity.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
 The data indicated that administering CT-996 to participants who are obese and do not have type 2 diabetes led to a clinically significant placebo-adjusted average weight reduction of -6.1% over a four-week period. Detailed study results will be disclosed at an upcoming medical conference.
The data indicated that administering CT-996 to participants who are obese and do not have type 2 diabetes led to a clinically significant placebo-adjusted average weight reduction of -6.1% over a four-week period. Detailed study results will be disclosed at an upcoming medical conference.
Obesity is a critical global health issue with numerous related conditions, including type 2 diabetes, cardiovascular disease, liver disease, and chronic kidney disease. Estimates suggest that over four billion individuals – approximately 50% of the world’s population – will be affected by obesity or be overweight by the year 2035.
"We are encouraged by the clinically significant weight reduction observed in individuals treated with CT-996, our oral GLP-1 therapy, which has the potential to aid patients in managing chronic weight and glycemic control," stated Levi Garraway, M.D., Ph.D., Chief Medical Officer and Head of Global Product Development. "Following the positive outcomes with CT-388, CT-996 signifies the second successful result from our expanding metabolic pipeline within a span of less than three months, offering both oral and injectable solutions to address a wide range of patient needs."
CT-996 was generally well-tolerated, exhibiting primarily mild to moderate gastrointestinal side effects, which align with the known safety profile of incretin drugs. No patients discontinued the study due to adverse effects from the treatment.
The study also revealed that blood levels of CT-996 remained stable during fasting and after a standardized high-fat meal, suggesting that the drug could be administered without regard to mealtime, thereby providing greater flexibility for patients.
Based on these findings, CT-996 is expected to be useful not only for glycemic control and initiating weight loss but also as a potential oral maintenance therapy following weight reduction achieved through injectable treatments.
CT-996 is an investigational GLP-1 receptor agonist taken once daily in oral form, being developed to manage both type 2 diabetes and obesity. Unlike the naturally occurring GLP-1 hormone, CT-996 is engineered to be a biased GLP-1 receptor agonist that selectively activates cAMP signaling while minimally engaging beta-arrestin. These specific signaling properties are anticipated to result in substantial glycemic control, significant weight loss, and favorable tolerance.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
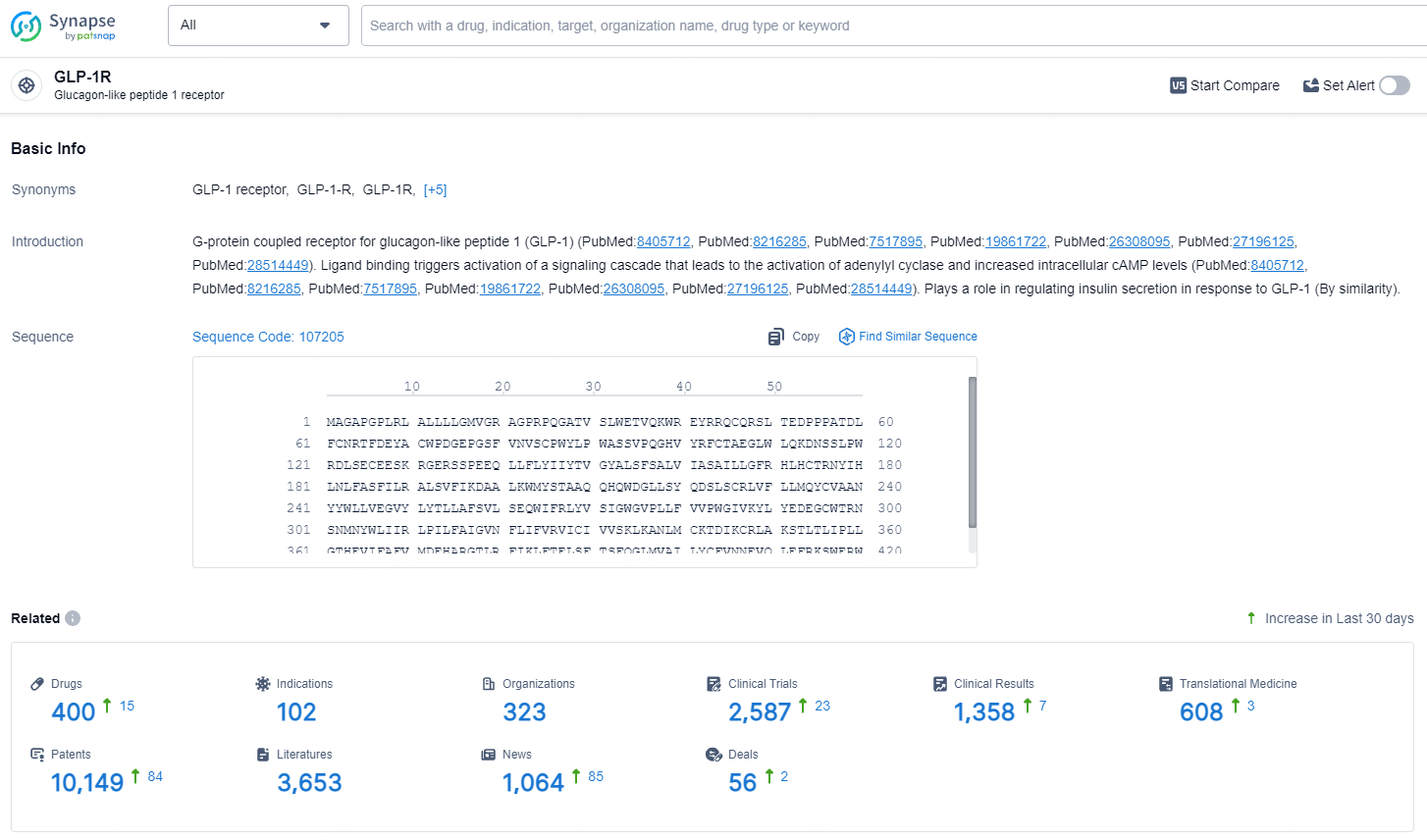
According to the data provided by the Synapse Database, As of July 22, 2024, there are 400 investigational drugs for the GLP-1 targets, including 102 indications, 323 R&D institutions involved, with related clinical trials reaching 2587, and as many as 10149 patents.
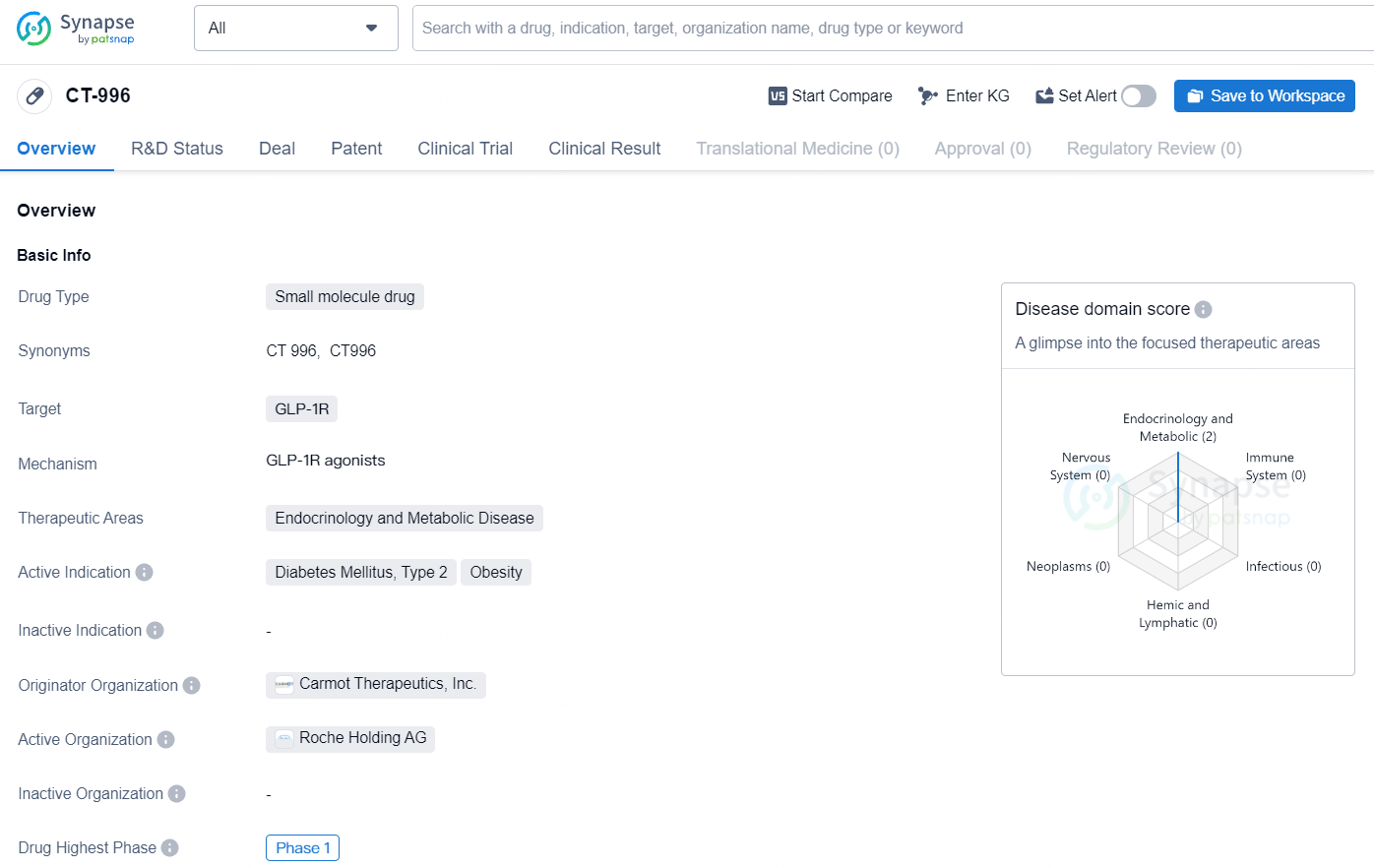
,CT-996 is a small molecule drug targeting the GLP-1R receptor, with a focus on addressing the therapeutic areas of endocrinology and metabolic disease. Its current development status at Phase 1 suggests that it is in the early stages of clinical evaluation, with the potential to offer new treatment options for Diabetes Mellitus, Type 2, and obesity. Further advancements in its development will be essential for determining its ultimate clinical utility and impact on the pharmaceutical market.