Is Ciltacabtagene autoleucel approved by the FDA?
Ciltacabtagene autoleucel received FDA approval on February 28, 2022. This milestone approval marks a significant advancement in the treatment options available for adults with relapsed or refractory multiple myeloma, offering new hope where traditional treatments have failed.
Mechanism of Action
Ciltacabtagene autoleucel is a form of CAR T-cell therapy, where T cells (a type of white blood cell) are extracted from the patient's blood through a process called leukapheresis. These T cells are then genetically modified to produce chimeric antigen receptors (CARs) that target and destroy cancerous cells when reintroduced into the patient's bloodstream. This targeted approach helps in reducing cancer burden and improving patient outcomes.
Usage and Administration
This medication is only available through a special program and must be administered under the supervision of trained healthcare professionals in authorized hospitals or clinics. The treatment process involves several steps:
- Leukapheresis: A procedure to collect T cells from the patient's blood.
- Genetic Modification: The collected T cells are modified outside the body to produce CARs.
- Pre-treatment: Patients receive chemotherapy to prepare their bodies for receiving the modified T cells.
- Infusion: The modified T cells (ciltacabtagene autoleucel) are then infused back into the patient through intravenous infusion.
Side Effects
Ciltacabtagene autoleucel treatment can lead to various side effects, including potentially serious conditions such as cytokine release syndrome (CRS). Symptoms of CRS include fever, chills, breathing difficulties, and rapid heartbeat, which require immediate medical attention. Other common side effects may include neurological symptoms like confusion, as well as infections and low blood cell counts.
Precautions and Warnings
Before starting treatment with ciltacabtagene autoleucel, patients must undergo comprehensive medical evaluation, including tests for neurologic, cardiac, hepatic, and renal function. Special precautions are necessary for patients with a history of neurologic disorders, breathing problems, or heart conditions. Women of childbearing potential must undergo pregnancy testing prior to treatment and use effective contraception during therapy.
Monitoring and Follow-up
Patients receiving ciltacabtagene autoleucel require close monitoring for potential adverse effects, including the risk of developing secondary cancers. Regular follow-up visits and blood tests are essential to assess treatment response and manage any complications that may arise.
Conclusion
Ciltacabtagene autoleucel (Carvykti) represents a groundbreaking therapy for adults with relapsed or refractory multiple myeloma, providing a targeted approach to cancer treatment through CAR T-cell technology. FDA approval in February 2022 underscores its efficacy and safety profile in addressing a critical unmet need in oncology. This therapy offers renewed hope and therapeutic options for patients facing advanced stages of multiple myeloma, paving the way for further advancements in cancer immunotherapy.
How to obtain the latest development progress of all drugs?
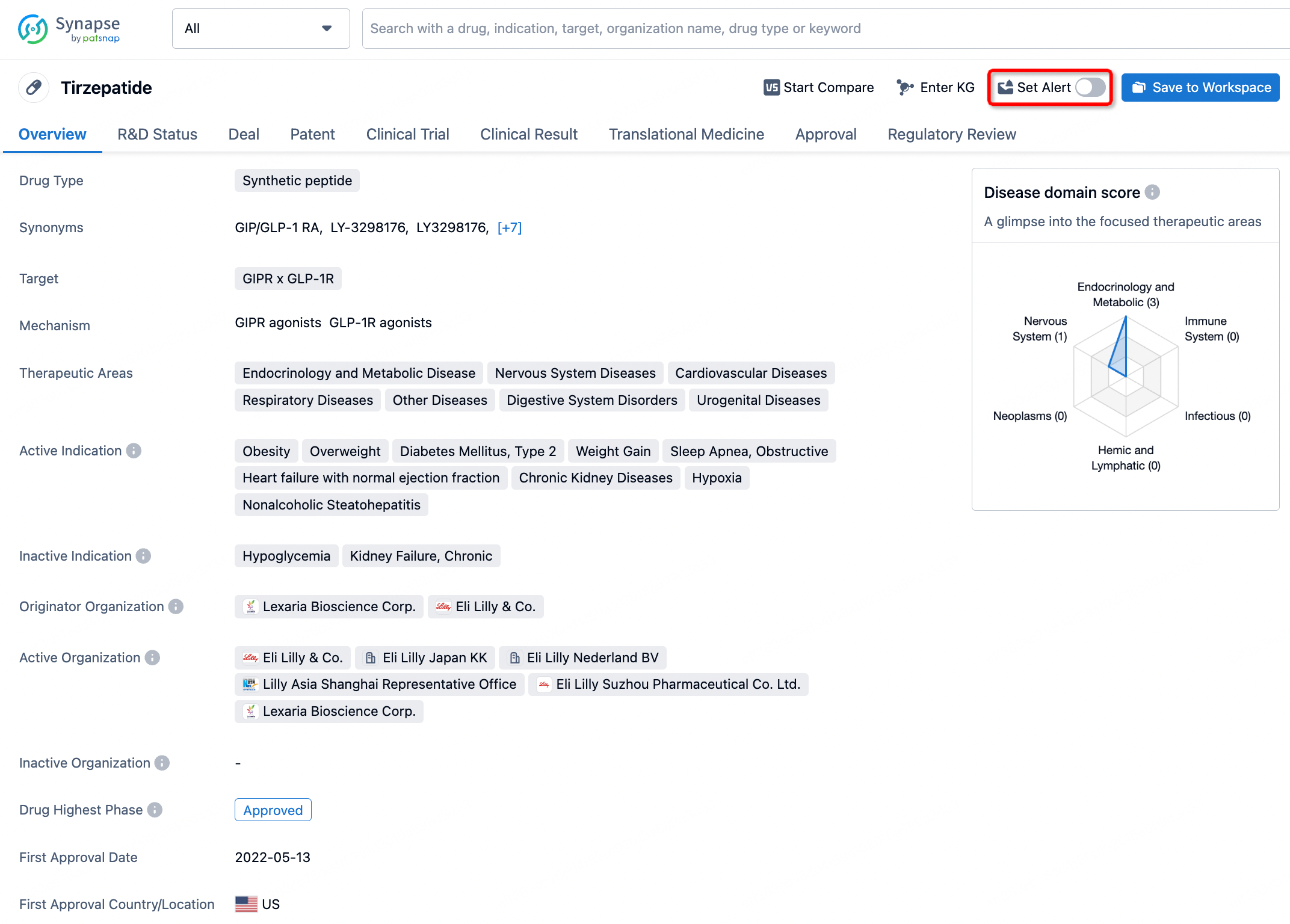
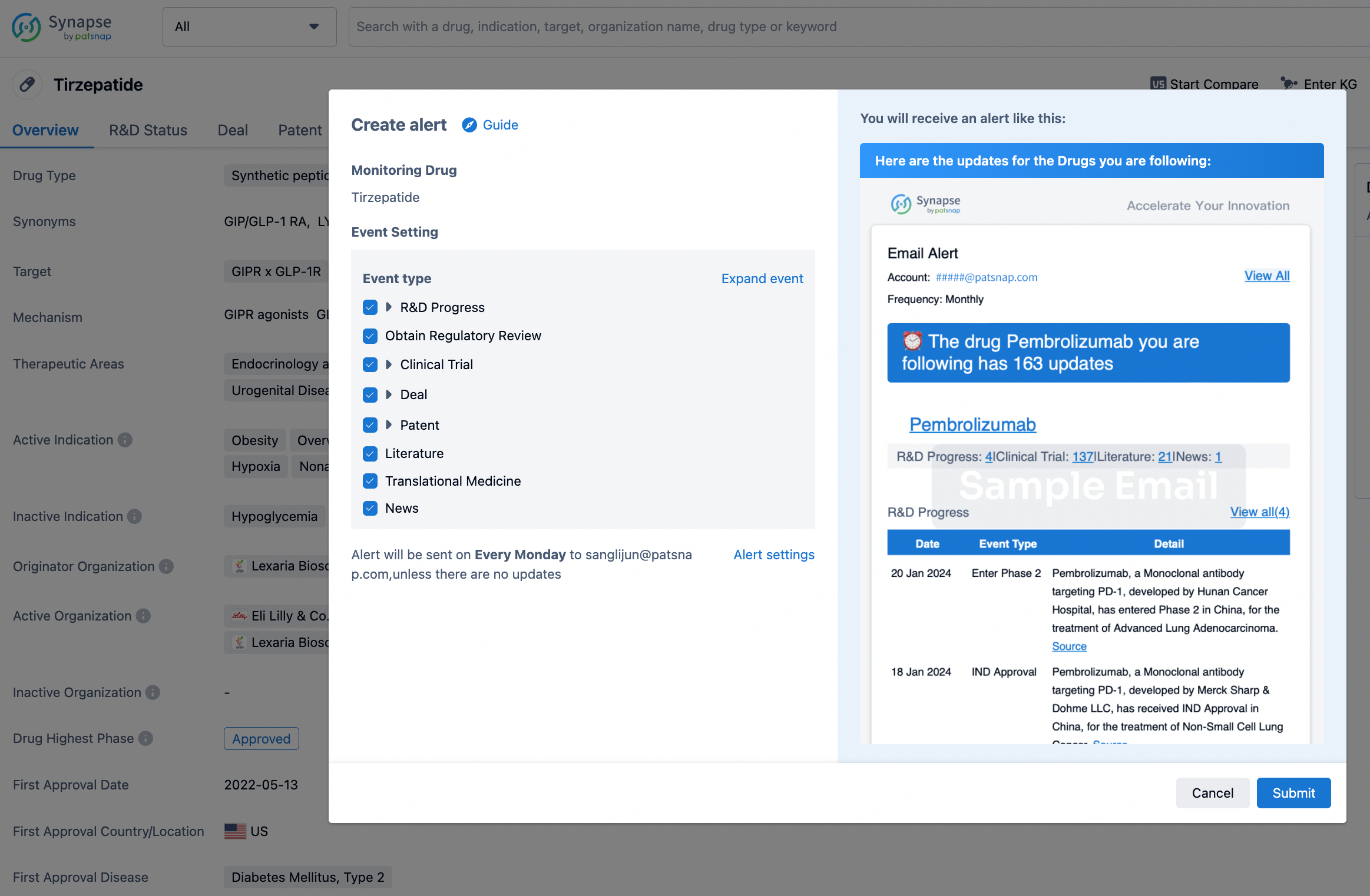
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!