Quickly Locating Your Target Sequence in Patent Claims Containing a Large Number of Antibody Sequences
In the vast sea of patents, pinpointing a target sequence can be daunting. Utilizing the Patsnap patent database, alongside its Bio Database, simplifies this process, rendering rapid and accurate results. Here's a step-by-step guide on how to leverage this powerful toolset:
Commence your journey by registering for a free Bio Database account, which can easily be achieved by clicking Patsnap Bio Sequence Database. Once registered, input your patent number, e.g., WO2018020476A1, into Patsnap's patent data search bar. Once located, open the patent to view its detailed page, where its disclosed rights can be viewed on the right-claim page. Then, click Sequence Assistant in the top right corner, enter the target sequence you want to find, such as SASYRYS, and click "Search Sequence," as shown in the figure below.
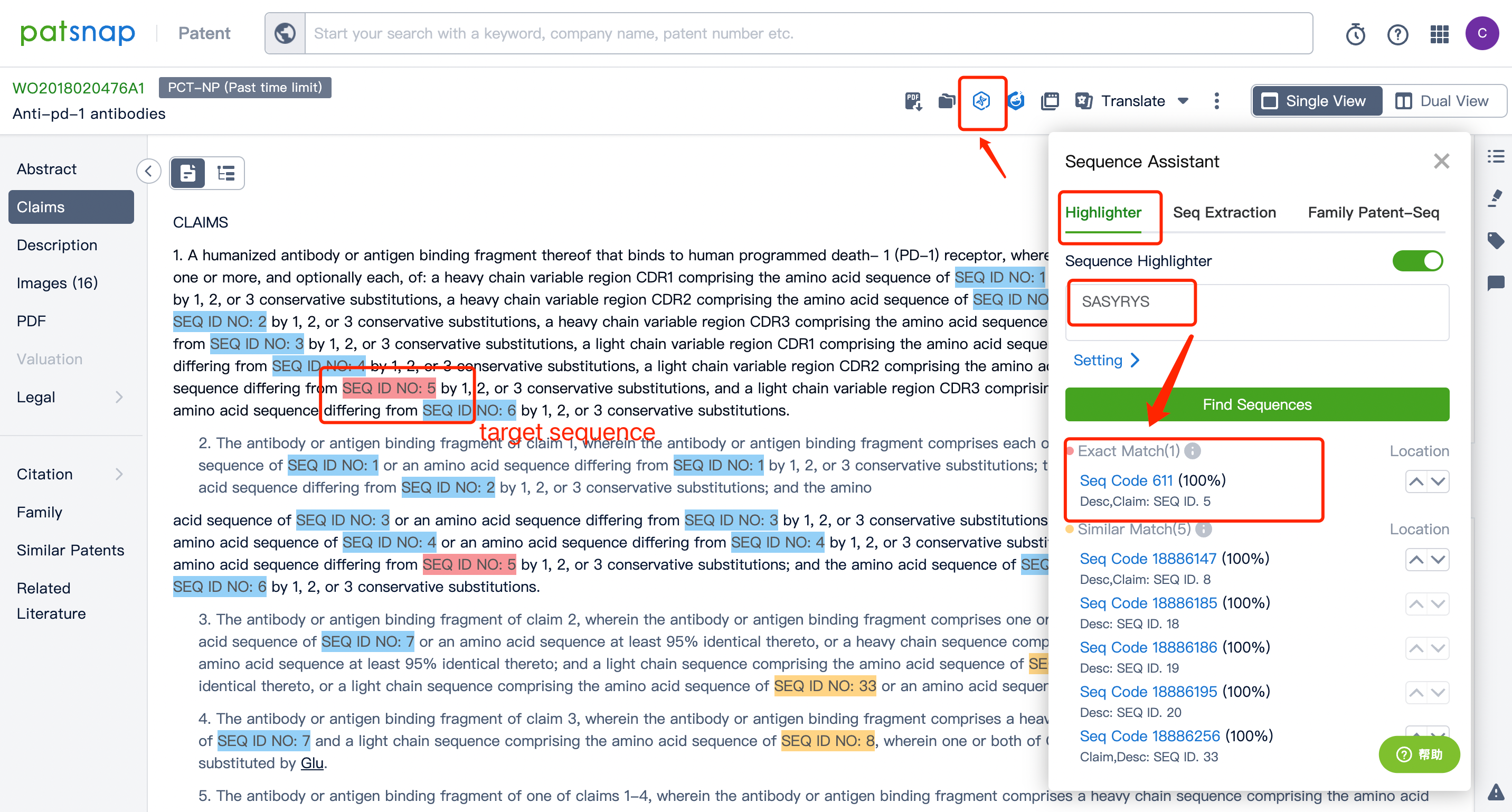
Toggle the "Sequence Highlight" feature on the right for an even more precise view. Fully matched sequences will be illuminated in red and partially matched in yellow, while the remaining patent sequences will appear in blue. This results in your target sequence, in this case, "SEQ ID NO: 5", being evident and easily located within the claim of rights, displaying all its positions.
It is important to note that Patsnap Bio is the most extensive sequence search platform for the Patsnap database. It incorporates AI with human-curated data for comprehensive handling of protein and nucleotide sequence data plucked from global patents, biological periodicals, and public repositories. Essential biological sequences are manually annotated, illuminating structural modifications to provide the most accurate sequence data and boost sequence retrieval efficiency.
Free registration is available for the Bio biological sequence database: https://bio.patsnap.com. Act now to expedite your sequence search tasks.




