Rallybio Reveals Early Data from Phase 1 Escalating Dose Trial for RLYB116, a Novel Subcutaneous Complement 5 Blocker
Rallybio Corporation has disclosed initial data from a Phase 1 trial that utilized a stepped approach to increase dosages for the investigational drug, RLYB116. This novel drug candidate, characterized by its extended duration of action and its administration in small volumes under the skin, functions as a suppressor of the C5, which is part of the complement system. The drug is under research for potential therapeutic benefit to individuals suffering from diseases driven by abnormal complement activation.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The initial Multiple Ascending Dose investigation of RLYB116 conducted a comprehensive review on its acceptability, safety profile, kinetic properties of the drug in the system, and its effects concerning disposition and action on the human body following administrations beneath the skin in a sample of healthy individuals. This exploratory phase studied the responses to RLYB116 over several administrations.
The research approach was flexible and was designed to modify in response to ongoing results. This particular study incorporated four distinct groups with each consisting of a dozen participants. Those in the study received RLYB116 or a non-effective substitute at dosages reaching 200 milligrams weekly. Participants underwent a treatment regimen covering a span of four weeks, succeeded by an observation period lasting ten weeks post-treatment.
Eric Watsky, M.D., the chief figure of the RLYB116 project at Rallybio, remarked on the promising results, noting significant reductions in free C5 due to RLYB116 and the successful levels attained through the subcutaneous delivery method. Dr. Watsky expressed confidence that recent advancements in the production process presented possibilities to refine the RLYB116 dosage and its acceptability, ultimately broadening its therapeutic potential across diverse illnesses involving complement system disorders.
Watsky also cited conclusions from marketing analysis that align with the company's vision, projecting a positive reception for a therapy that promises to be both efficacious and manageable on a weekly basis, particularly one that patients can self-deliver conveniently using an autoinjector.
According to the initial findings from this Phase 1 study, progress in the production methods for RLYB116 has reached a point where it's feasible for Rallybio to consider its application in clinical patient trials.
Choosing a strategic approach, the firm has elected not to immediately progress into a Phase 2 trial for gMG. Instead, the focus will remain on refining RLYB116's production. The expectation is that further enhancements to manufacturing will facilitate the use of the drug at stronger dosages while maintaining ease of administration and requiring smaller quantities for each subcutaneous injection.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
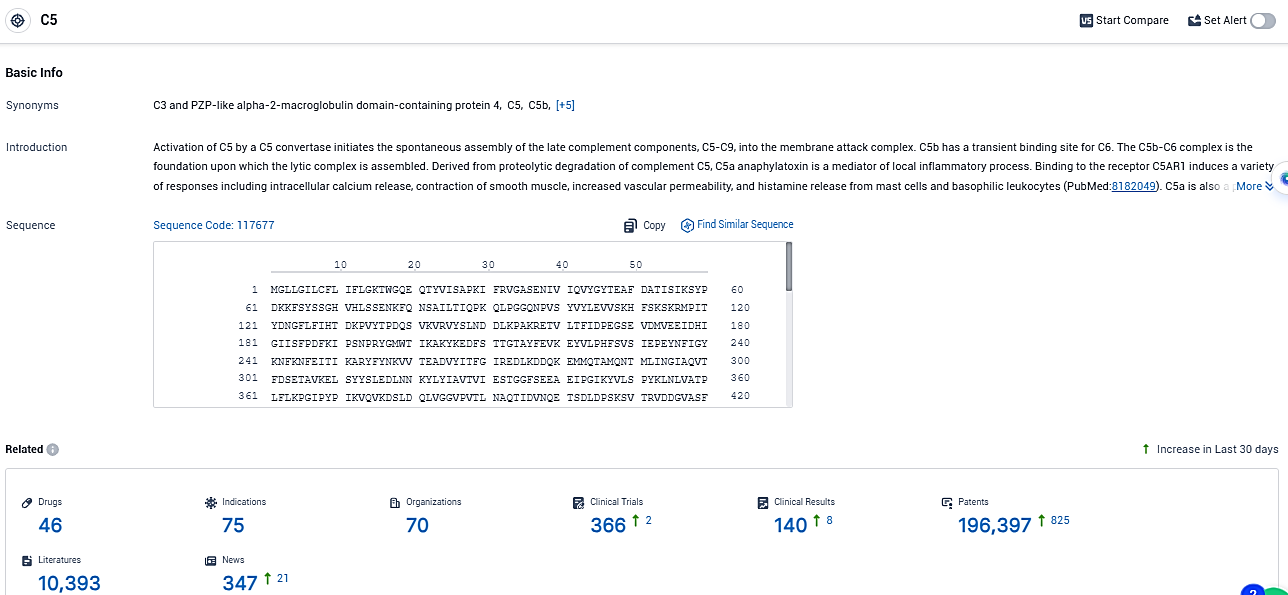
According to the data provided by the Synapse Database, As of December 28, 2023, there are 46 investigational drugs for the C5 target, including 75 indications, 70 R&D institutions involved, with related clinical trials reaching 366, and as many as 196397 patents.
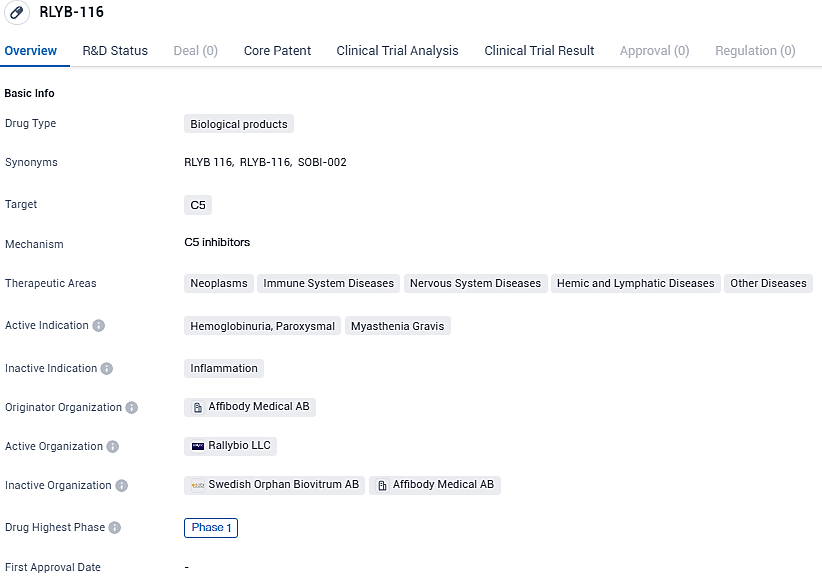
RLYB-116 targets C5, a protein involved in the complement system of the immune system. The therapeutic areas that RLYB-116 aims to address include neoplasms, immune system diseases, nervous system diseases, hemic and lymphatic diseases, and other diseases. Specifically, the drug is being investigated for its potential use in treating hemoglobinuria, paroxysmal and myasthenia gravis.