First participant receives dose in Tiziana Biotech's early-stage trial for nasal administered Foralumab targeting MS
Tiziana Life Sciences Ltd., an enterprise engaged in crafting innovative therapies focused on neuro-immunomodulation, recently declared the commencement of treatment on the initial participant in a Phase 2a clinical trial. This study is designed to evaluate the efficacy and safety of two different administration levels of the intranasal drug foralumab, as well as a placebo, in individuals diagnosed with secondary-progressive multiple sclerosis that is currently non-active.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Several research sites, totaling six, have been selected to participate in a randomized, double-blind trial with a placebo comparison, which will include a maximum of 18 individuals for each treatment group. The pivotal outcome measure of the study is the alteration in microglial activation, ascertained via PET imaging. Assessment procedures during the trial encompass the Expanded Disability Status Scale for neurological impairment, Quality of Life evaluations, and the Modified Fatigue Impact Scale to gauge aspects fundamental to the daily experiences of those involved.
Gabriele Cerrone, the Chairman, interim CEO and the originator of Tiziana Life Sciences, expressed confidence that the initial patient dosing showcased Tiziana's dedication to its objectives and the prospects to promote their proprietary intranasal anti-CD3 monoclonal antibody, foralumab, with innovative imaging techniques and endpoints pertinent to clinical practice. The team is hopeful that their ongoing research will provide an alternative treatment avenue for individuals battling this incapacitating condition. At present, no FDA-sanctioned treatments exist for non-active secondary progressive multiple sclerosis (na-SPMS).
Matthew W. Davis, MD, RPh, who holds the positions of Chief Operating Officer and Chief Medical Officer at Tiziana, announced his satisfaction with the commencement of dosing the initial study participant. He highlighted the commitment of his team to adhere to the projected timelines diligently. They aim to expedite participant enrollment and are expecting preliminary study outcomes by the fourth quarter of 2024. He envisioned that the findings of this investigation would provide significant insights into refining the treatment approach for na-SPMS, specifically regarding the potential to decrease MFIS scores.
The function of activated T cells is crucial within the context of inflammatory responses. Foralumab represents the sole fully human anti-CD3 monoclonal antibody, designed to bind with the T cell receptor. It mitigates inflammatory processes by modulating the activity of T cells, curbing the active characteristics observed in diverse populations of immune cells. This immunomodulatory effect of Foralumab has been observed in patients dealing with COVID, multiple sclerosis, and even in individuals without any disease conditions.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
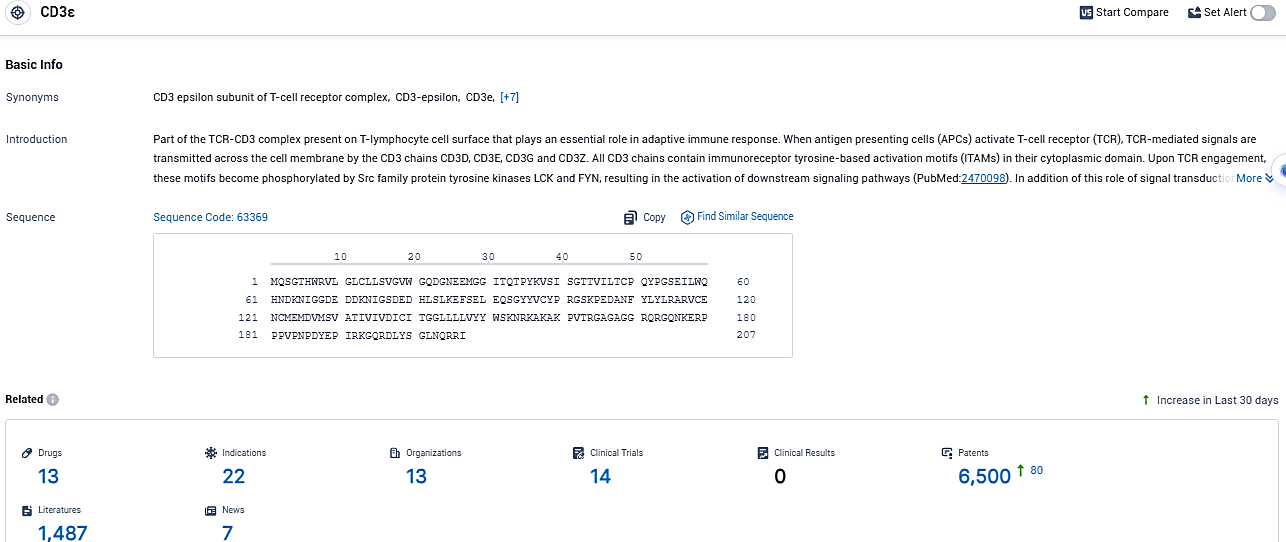
According to the data provided by the Synapse Database, As of December 28, 2023, there are 13 investigational drugs for the CD3ε target, including 22 indications, 13 R&D institutions involved, with related clinical trials reaching 14, and as many as 6500 patents.
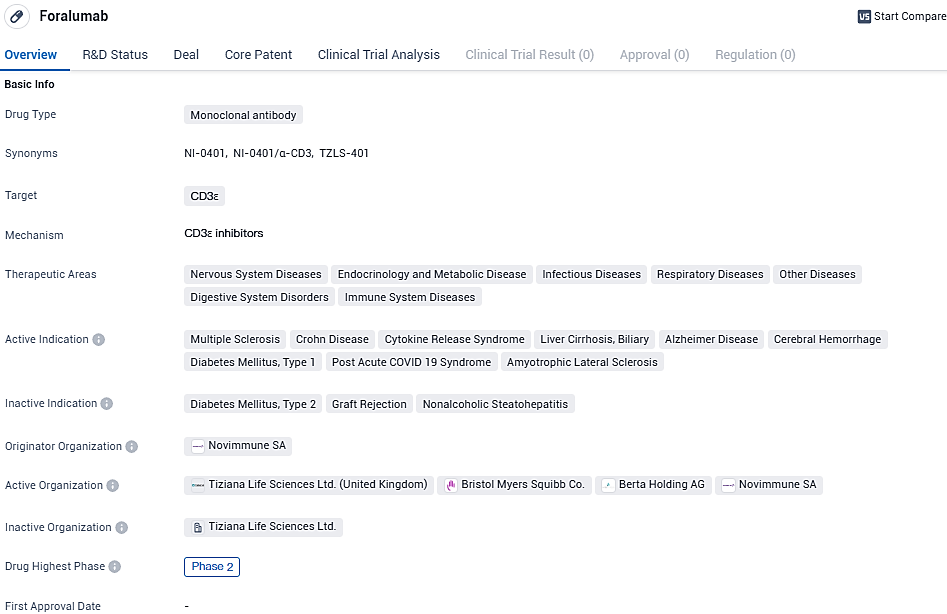
Foralumab has a wide range of therapeutic areas and active indications, including diseases of the nervous system, endocrinology and metabolism, infectious diseases, respiratory diseases, digestive system disorders, and immune system diseases. While it has reached Phase 2 of clinical development, further research is required to fully understand its potential and determine its safety and efficacy in treating these conditions.