Reddy’s Labs introduces Versavo® (bevacizumab) to the UK market
Dr. Reddy’s Laboratories Ltd., an international enterprise specializing in pharmaceuticals, has officially declared the introduction of its drug Versavo® (bevacizumab) to the UK market. The product, Versavo®, is a biosimilar to Avastin®, formulated for the management of various cancer forms, such as metastatic colorectal cancer, progressive non-squamous non-small cell lung cancer, recurring glioblastoma, metastatic renal cell carcinoma, advanced cervical cancer, ovarian cancer, and metastatic breast cancer.
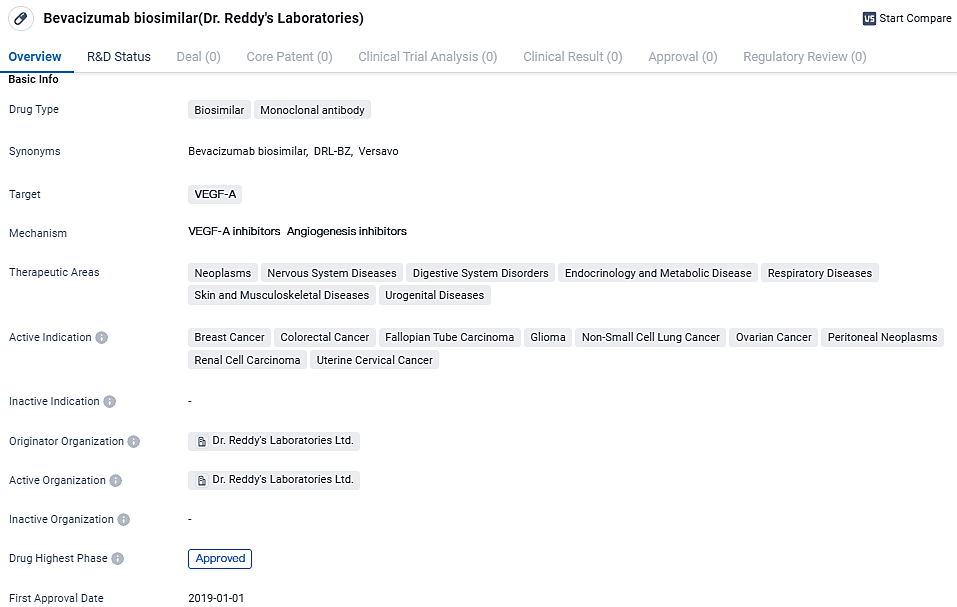
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Dr. Reddy's has successfully obtained approval for and introduced their initial biosimilar, Versavo®, in the United Kingdom. This product is accessible in 100mg and 400mg concentrations, each contained in single-use vials for administration.
The pharmaceutical company first unveiled Versavo® on the Indian market in 2019 before rolling it out to several other regions, including Thailand, Ukraine, Nepal, and Jamaica, maintaining the Versavo® branding. However, in Colombia, the biosimilar is marketed under the name Persivia®.
Global Head of Biologics at Dr. Reddy's, Dr. Jayanth Sridhar, articulated the significance of the launch: "Introducing Versavo® in a stringently regulated market is a testament to our proficiency in the global clinical development of biosimilars of superior quality. As a versatile therapeutic choice for diverse cancer types, Versavo® is pivotal. This move amplifies our dedication to enhancing patient access to vital biosimilars and underscores our deepening engagement with oncology treatment solutions."
Versavo® is a bevacizumab biosimilar developed by Dr. Reddy’s Laboratories. Bevacizumab marks a pioneering advancement in the fight against cancer with its ability to inhibit the proliferation of metastatic conditions. With its design as a humanized monoclonal antibody, it specifically engages with the human vascular endothelial growth factor (VEGF), effectively curbing new blood vessel formation and consequently, tumor expansion.
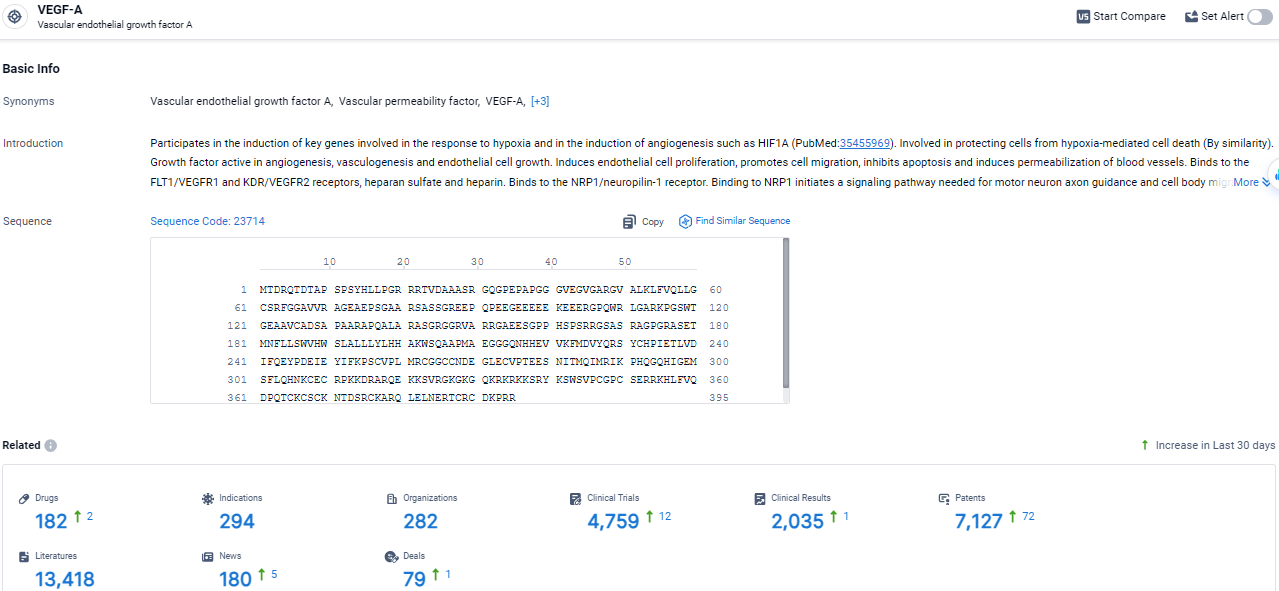
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of March 21 2024, there are 182 investigational drugs for the VEGF-A target, including 294 indications, 282 R&D institutions involved, with related clinical trials reaching 4759, and as many as 7127 patents.
Bevacizumab biosimilar is a monoclonal antibody drug that targets VEGF-A. It has been approved for the treatment of various cancers and other diseases. The drug offers a biosimilar alternative to the originator drug, potentially providing cost-effective treatment options for patients.