Actinium-Based PSMA Radioconjugate Deal Targets Prostate Cancer Treatment and Development
Fusion Pharmaceuticals Inc., a firm in the clinical phase specializing in oncology, has announced its commitment to advance novel radioconjugate therapies aimed at targeted treatments. The company has confirmed that it has signed a formal acquisition agreement with pharmaceutical giant AstraZeneca.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
This recent acquisition signifies an important stride for AstraZeneca in its pursuit to revolutionize oncological healthcare by substituting conventional treatments such as chemotherapy and radiotherapy with highly specific therapies.
Radioconjugates (RCs) have surfaced as a groundbreaking approach in the domain of cancer care over the past few years, showcasing significant promise. These pharmaceutical agents transport a radioactive isotope straight to the malignant cells, utilizing highly specific vectors that include antibodies, peptides, or small molecular structures. This strategy offers multiple potential benefits in comparison to traditional radiotherapy methods, such as reducing collateral damage to normal cells and being able to treat neoplasms that are inaccessible to standard external beam radiation.
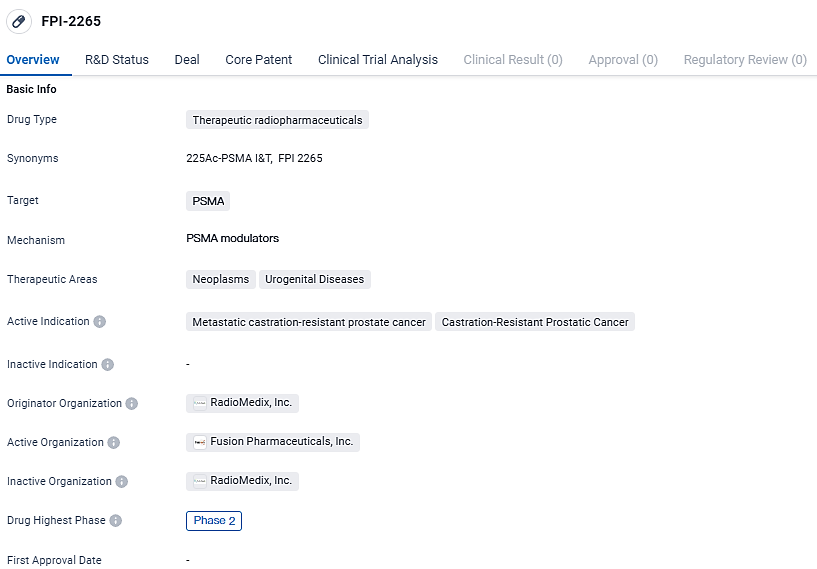
By absorbing Fusion's suite of RCs into its portfolio, AstraZeneca fortifies its position in the oncological sector. This move encompasses Fusion’s diverse RC collection, notably featuring their lead candidate, FPI-2265, an investigational therapeutic for individuals with metastatic castration-resistant prostate cancer (mCRPC). FPI-2265 is designed to home in on the prostate-specific membrane antigen (PSMA), which displays increased presence in mCRPC and is being evaluated in a Phase 2 clinical trial.
The integration of Fusion amplifies AstraZeneca’s capacity in pioneering research, development, manufacturing, and distribution of actinium-tethered RCs. The move also reinforces AstraZeneca's engagement and dedication to the Canadian healthcare landscape.
Expressing his views on the consolidation, Mohit Rawat, Fusion's President and Chief Business Officer, remarked: “Fusion has carved a unique niche within the burgeoning field of radioconjugates, assembling a top-tier cadre of experts and the necessary infrastructure to expedite the development of these critical treatments for oncology patients. We are eager to augment our work and reshape cancer treatment modalities through our intensified efforts with AstraZeneca. This collaboration provides a stimulating platform for the Fusion collective."
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
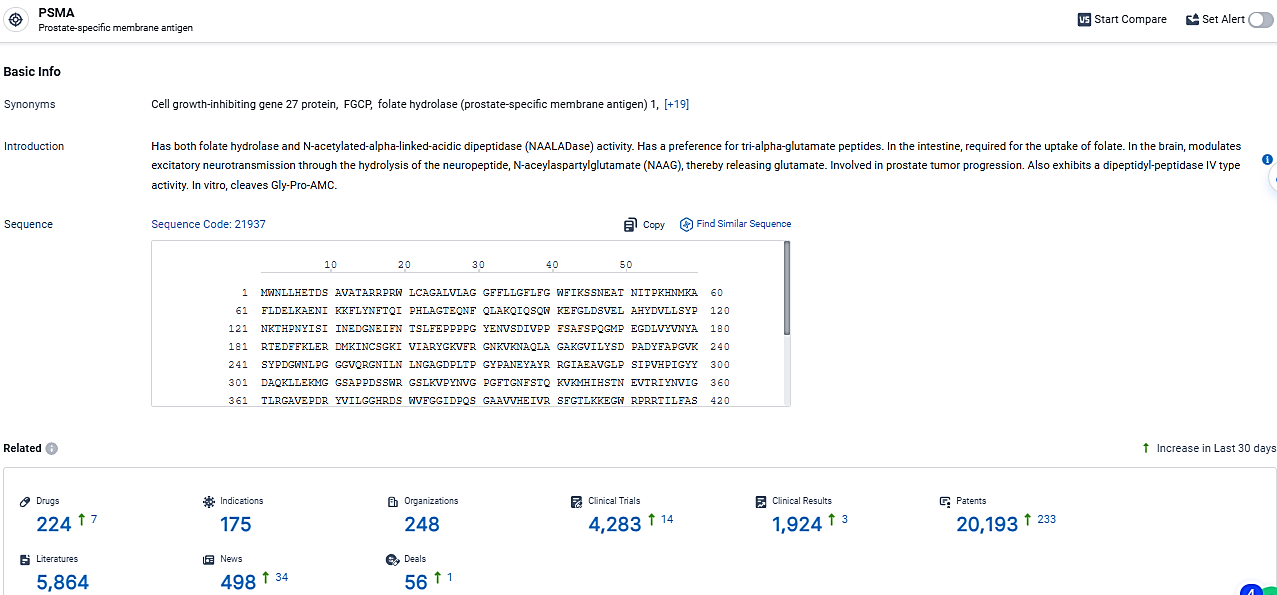
According to the data provided by the Synapse Database, As of March 20 2024, there are 224 investigational drugs for the PSMA target, including 175 indications, 248 R&D institutions involved, with related clinical trials reaching 4283, and as many as 20193 patents.
FPI-2265 targets PSMA and is primarily used in the treatment of metastatic castration-resistant prostate cancer and castration-resistant prostatic cancer. The drug has reached Phase 2 of development, indicating promising progress in its clinical evaluation. Further research and testing will be necessary to determine its potential as a treatment option for patients with these conditions.