Remix Therapeutics Initiates Phase 1 Trials of REM-422 for ACC and AML/MDS Treatment
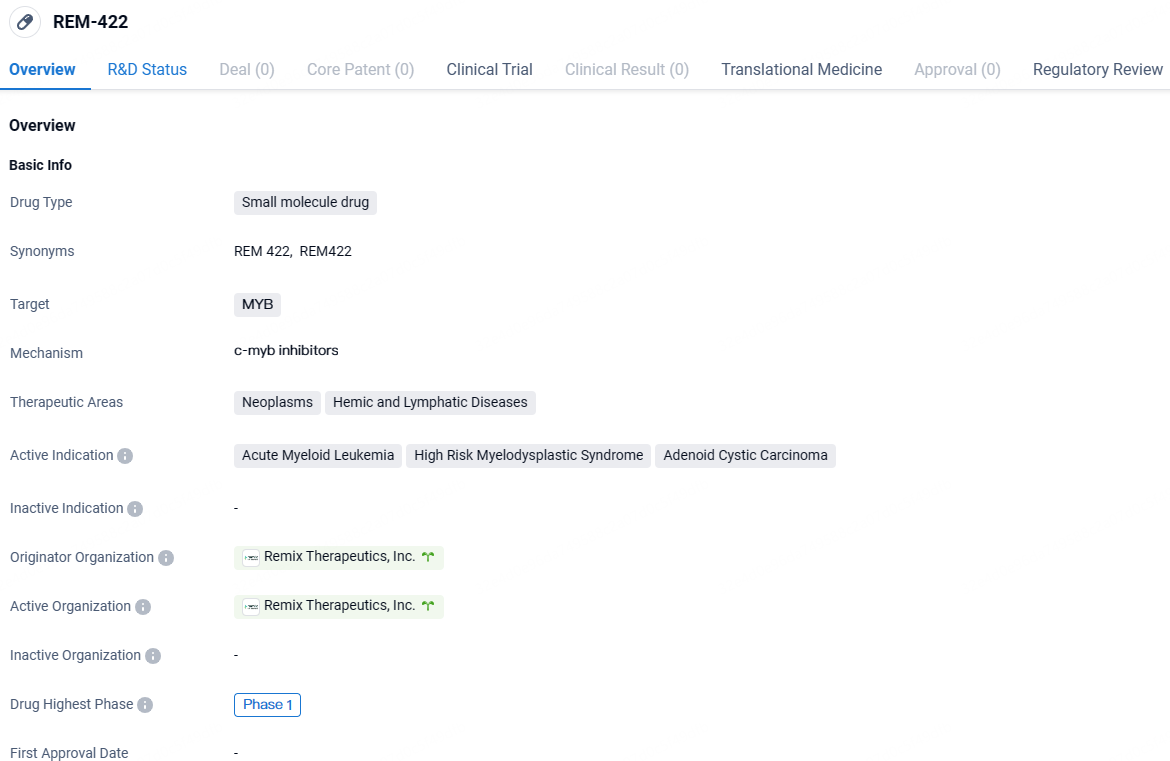
Remix Therapeutics, a company in the clinical-stage of biotech development focusing on small molecule treatments that adjust RNA processing to combat primary disease causes, has disclosed the initiation and dosing of the first participants in two separate Phase 1 studies. These trials are evaluating the efficacy of REM-422, a pioneering MYB mRNA degrader, in treating recurring or metastatic adenoid cystic carcinoma as well as acute myeloid leukemia/high-risk myelodysplastic syndromes.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Peter Smith, Ph.D., Co-Founder and CEO of Remix, announced a pivotal development regarding REM-422, originating from the REMaster discovery platform. "Initiating clinical trials with REM-422 is a crucial advancement for Remix," he remarked. "Initial safety and pharmacokinetic evaluations in patients have been encouraging, and we're optimistic about further exploring REM-422's potential to inhibit MYB, an oncogenic transcription factor traditionally deemed challenging to target, benefiting individuals with ACC and AML/MDS."
The ongoing investigations consist of two Phase 1 open-label, non-randomized, multicenter studies focusing on REM-422 for treating patients with either recurrent or metastatic ACC or those with relapsed or refractory AML and high-risk MDS. Each study is structured around a Dose Escalation Phase followed by a Dose Expansion Phase to deepen our understanding of the drug's safety, pharmacokinetics/pharmacodynamics (PK/PD), and its effect on tumors.
MYB plays a crucial role as an oncogenic promoter in various solid and hematological cancers, including ACC and AML. REM-422, a robust, selective, oral mRNA degrader, targets MYB mRNA for degradation, decreasing MYB protein levels and thus impeding tumor growth in models reliant on MYB.
"Those affected by metastatic ACC are in urgent need of viable treatment avenues, underscored by our receiving an orphan drug designation," explained Chris Bowden, M.D., Chief Medical Officer at Remix. "REM-422, aimed at correcting the abnormal MYB activity at the core of this condition, holds promise. We are keen to assess further its potential as a primary treatment for metastatic ACC while also evaluating its safety and effectiveness in managing AML and high-risk MDS, where MYB is a key factor in disease progression."
The U.S. FDA grants an Orphan Drug Designation to experimental treatments developed for rare health disorders affecting fewer than 200,000 U.S. residents.
REM-422 is a groundbreaking, selective, oral small molecule that degrades mRNA leading to MYB mRNA and protein reduction. It selectively integrates poison exons into the mRNA, triggering a decay mechanism that severely limits protein synthesis. By targeting MYB dysregulation upstream of protein formation, REM-422 addresses a fundamental aspect of cancer development.
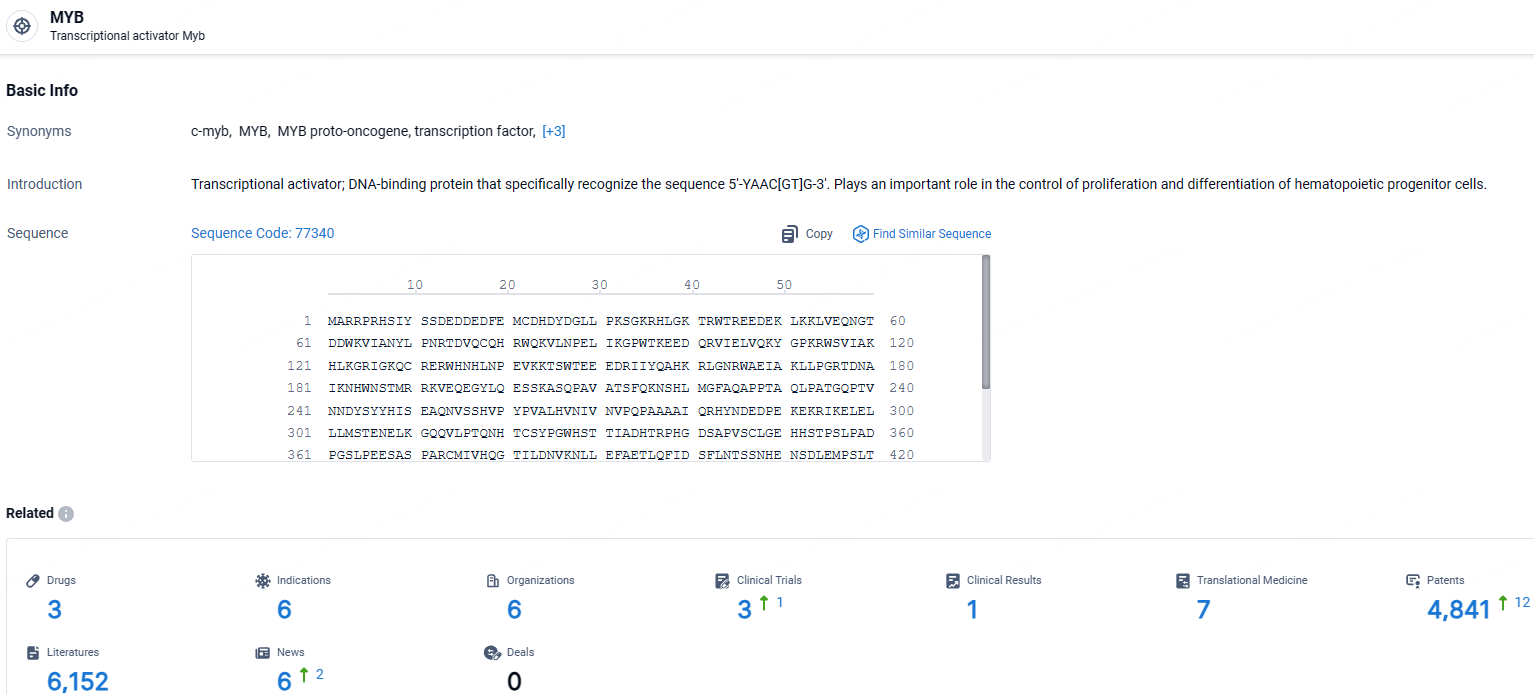
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of May 9, 2024, there are 3 investigational drugs for the MYB targets, including 6 indications, 6 R&D institutions involved, with related clinical trials reaching 3, and as many as 4841 patents.
REM-422 shows promise as a potential treatment option for patients with acute myeloid leukemia, high-risk myelodysplastic syndrome, and adenoid cystic carcinoma. However, further clinical trials and regulatory approvals will be necessary to determine its efficacy and safety profile. The drug's progression to Phase 1 indicates that it has passed initial preclinical testing and has shown potential in early studies. As the development of REM-422 continues, it will be important to closely monitor its progress and evaluate its potential impact on patients in need of effective treatments for these diseases.