Revefenacin Unveiled: A Detailed Overview of its Revolutionary R&D Breakthroughs, Mechanisms of Action, and Drug Target
Revefenacin's R&D Progress
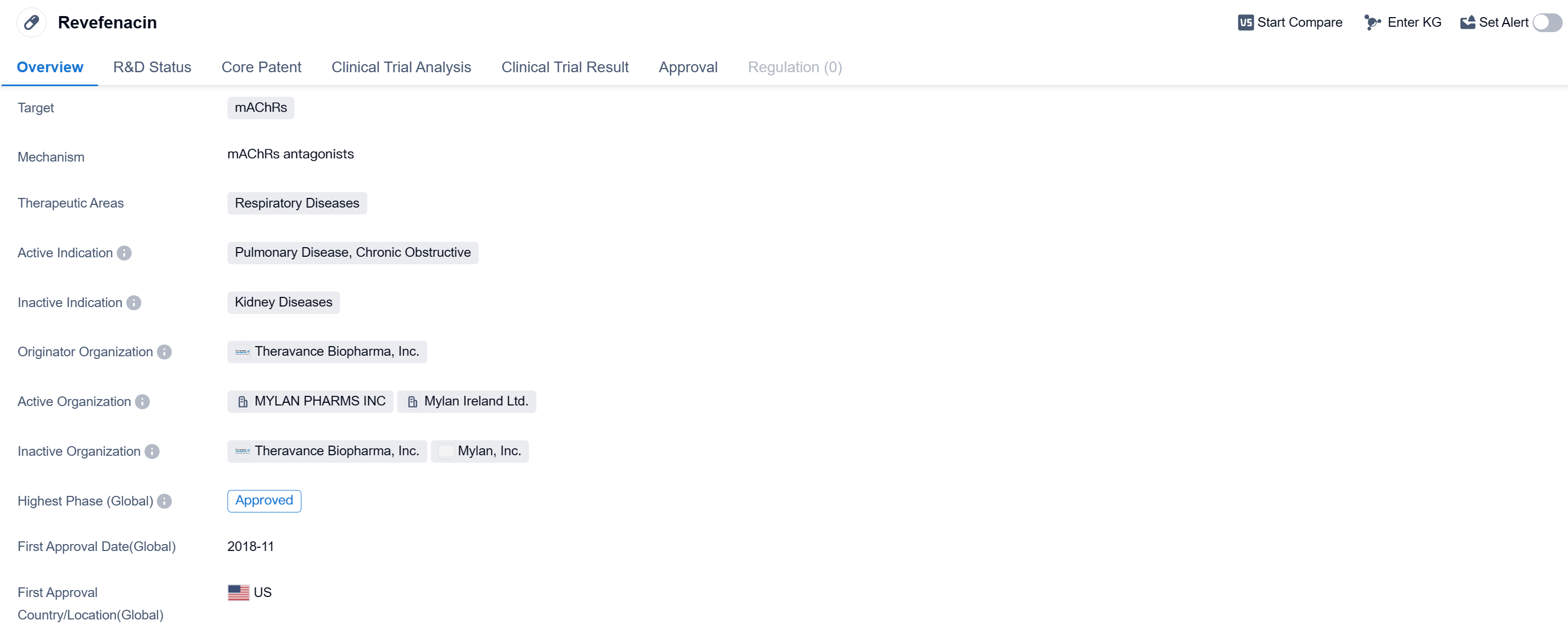
Revefenacin is a small molecule drug that falls under the therapeutic area of respiratory diseases. It specifically targets mAChRs (muscarinic acetylcholine receptors) and has been approved for the treatment of pulmonary disease, specifically chronic obstructive pulmonary disease (COPD). The drug was developed by Theravance Biopharma, Inc., a pharmaceutical organization specializing in the field of biomedicine.
Revefenacin received its first approval in the United States in November 2018, making it available for use in the country.
As a small molecule drug, Revefenacin is designed to interact with specific receptors in the body, in this case, mAChRs. By targeting these receptors, the drug aims to provide therapeutic benefits for patients suffering from respiratory diseases, particularly COPD. COPD is a chronic condition characterized by airflow limitation and is often associated with symptoms such as shortness of breath, coughing, and wheezing. Revefenacin offers a potential treatment option for individuals with this condition, helping to alleviate symptoms and improve overall lung function.
Theravance Biopharma, Inc. is the originator organization behind Revefenacin. This pharmaceutical company specializes in the development of innovative therapies for various diseases, with a particular focus on respiratory conditions. Their expertise in the field of biomedicine has led to the successful development and approval of Revefenacin, providing a valuable addition to the treatment options available for patients with COPD.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Revefenacin: mAChRs antagonists
mAChRs antagonists refers to muscarinic acetylcholine receptor antagonists. Muscarinic acetylcholine receptors (mAChRs) are a type of G-protein coupled receptors found in the central and peripheral nervous systems. They are involved in various physiological processes, including regulation of heart rate, smooth muscle contraction, glandular secretion, and neurotransmitter release.
Antagonists of mAChRs are drugs or compounds that bind to these receptors and block their activation by acetylcholine or other agonists. By blocking the action of acetylcholine at mAChRs, antagonists can inhibit the parasympathetic nervous system, leading to effects such as decreased heart rate, relaxation of smooth muscles, and decreased glandular secretion.
mAChRs antagonists have therapeutic applications in several medical conditions. For example, they can be used to treat overactive bladder by reducing bladder muscle contractions. They are also used in the management of chronic obstructive pulmonary disease (COPD) to help relax the airway smooth muscles and improve airflow. Additionally, mAChRs antagonists are sometimes prescribed to alleviate symptoms of gastrointestinal disorders, such as irritable bowel syndrome.
It is important to note that mAChRs antagonists can have side effects due to their broad effects on various organ systems. These side effects may include dry mouth, blurred vision, constipation, urinary retention, and cognitive impairment. Therefore, the use of mAChRs antagonists should be carefully monitored and prescribed by healthcare professionals.
Drug Target R&D Trends for Revefenacin
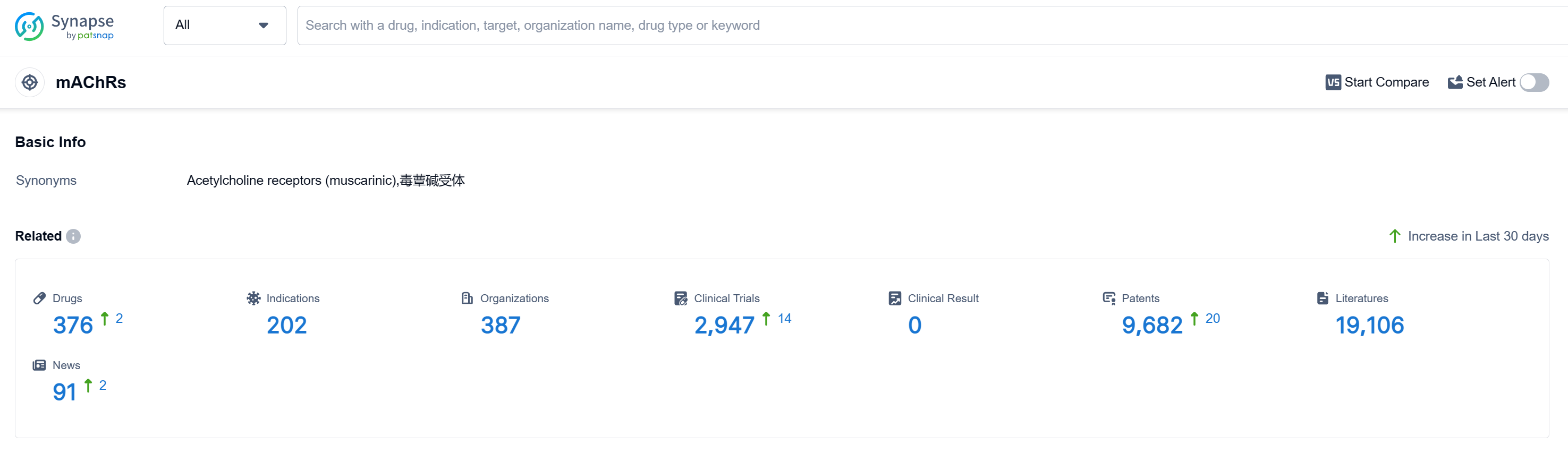
According to Patsnap Synapse, as of 10 Sep 2023, there are a total of 376 mAChRs drugs worldwide, from 387 organizations, covering 202 indications, and conducting 2947 clinical trials.
The analysis of target mAChRs reveals a competitive landscape with multiple companies actively developing drugs. Pfizer Inc., C.H. Boehringer Sohn AG & Co. KG, Santen Pharmaceutical Co., Ltd., AstraZeneca PLC, and Astellas Pharma, Inc. have shown significant growth and R&D progress. Approved indications cover a wide range of therapeutic areas, indicating the potential of mAChR-targeting drugs in treating various diseases. Small molecule drugs dominate the development of drugs targeting mAChRs, but other drug types, including biosimilars, are also being explored. China, along with other countries/locations such as Japan, the United States, and the European Union, is at the forefront of development. The future development of target mAChRs holds promising opportunities for innovative drugs and intense competition in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Revefenacin is a small molecule drug developed by Theravance Biopharma, Inc. for the treatment of respiratory diseases, specifically COPD. It targets mAChRs and has received approval in the United States. This drug offers potential benefits for individuals suffering from COPD, helping to manage symptoms and improve lung function. Its approval represents a significant milestone in the field of biomedicine and provides a valuable treatment option for patients in need.