Unleashing the Power of Risedronate sodium: A Comprehensive Review on R&D Breakthroughs, Action Mechanisms, and Drug Target
Risedronate sodium's R&D Progress
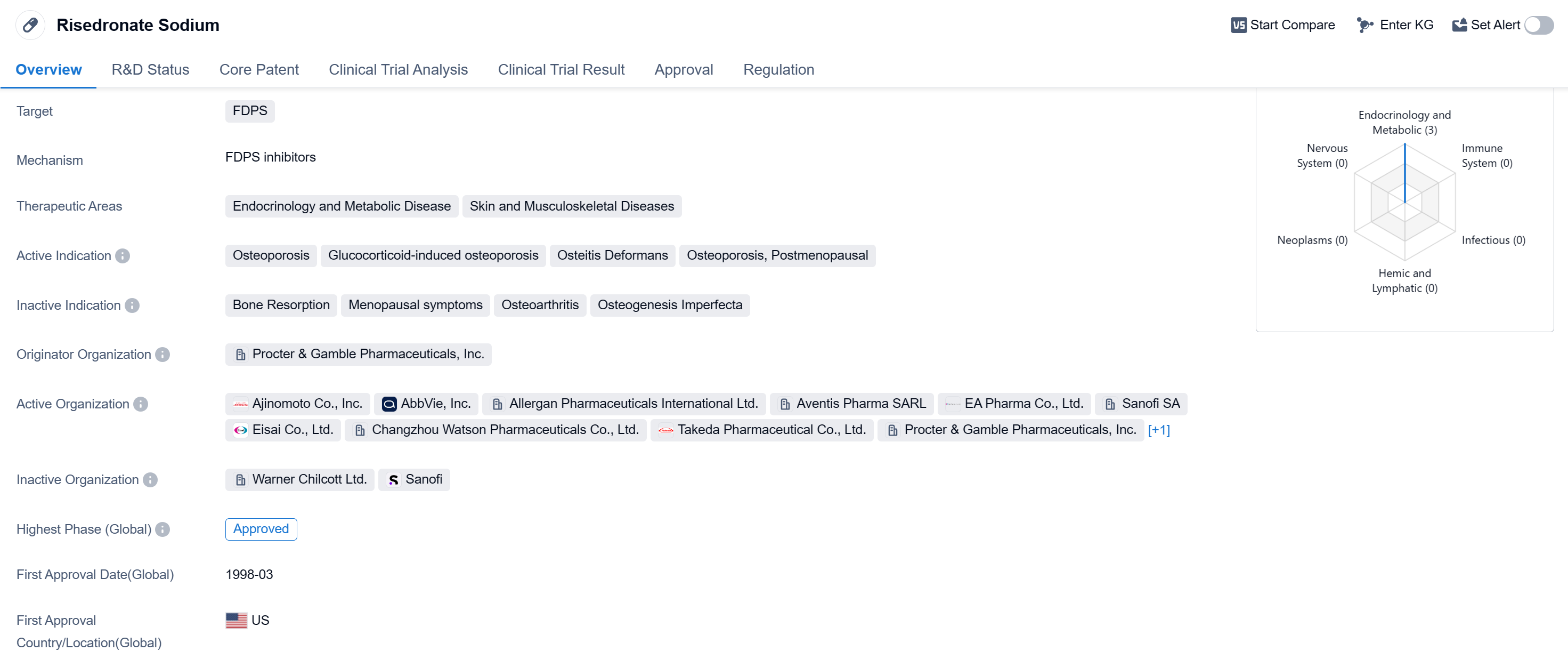
Risedronate Sodium is a small molecule drug that primarily targets FDPS. It is used in the treatment of various conditions related to endocrinology and metabolic diseases, as well as skin and musculoskeletal diseases. The drug is indicated for the treatment of osteoporosis, including glucocorticoid-induced osteoporosis, osteitis deformans, and postmenopausal osteoporosis.
The drug was developed by Procter & Gamble Pharmaceuticals, Inc., and it received its first approval in the United States in March 1998. Risedronate Sodium has since been approved in several countries globally, making it available for patients worldwide.
Risedronate Sodium is classified as an orphan drug, which means it is intended to treat rare diseases or conditions that affect a small number of patients. This designation provides certain incentives to pharmaceutical companies to develop drugs for these conditions.
As a small molecule drug, Risedronate Sodium is likely to have a well-defined chemical structure and can be easily synthesized in a laboratory setting. This characteristic may contribute to its stability and ease of manufacturing.
The drug's primary target, FDPS, is an enzyme involved in the biosynthesis of isoprenoid compounds. By inhibiting FDPS, Risedronate Sodium may help regulate the metabolic processes associated with the therapeutic areas it targets.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Risedronate sodium: FDPS inhibitors
FDPS inhibitors are a type of drug that target and inhibit the enzyme called farnesyl diphosphate synthase (FDPS). FDPS is an important enzyme involved in the mevalonate pathway, which is responsible for the production of cholesterol and other isoprenoid compounds.
From a biomedical perspective, FDPS inhibitors are primarily used in the treatment of certain types of cancer, particularly those that are dependent on the mevalonate pathway for their growth and survival. By inhibiting FDPS, these drugs disrupt the production of isoprenoid compounds that are essential for cancer cell proliferation and survival. This can help slow down or stop the growth of cancer cells and potentially lead to tumor regression.
FDPS inhibitors have shown promise in the treatment of various cancers, including breast, prostate, and lung cancer. They are often used in combination with other anticancer agents to enhance their effectiveness. However, like any medication, FDPS inhibitors can have side effects, including gastrointestinal disturbances, liver toxicity, and bone marrow suppression.
In summary, FDPS inhibitors are drugs that target the enzyme FDPS and are used in the treatment of certain cancers. They work by disrupting the production of isoprenoid compounds, which are essential for cancer cell growth and survival.
Drug Target R&D Trends for Risedronate sodium
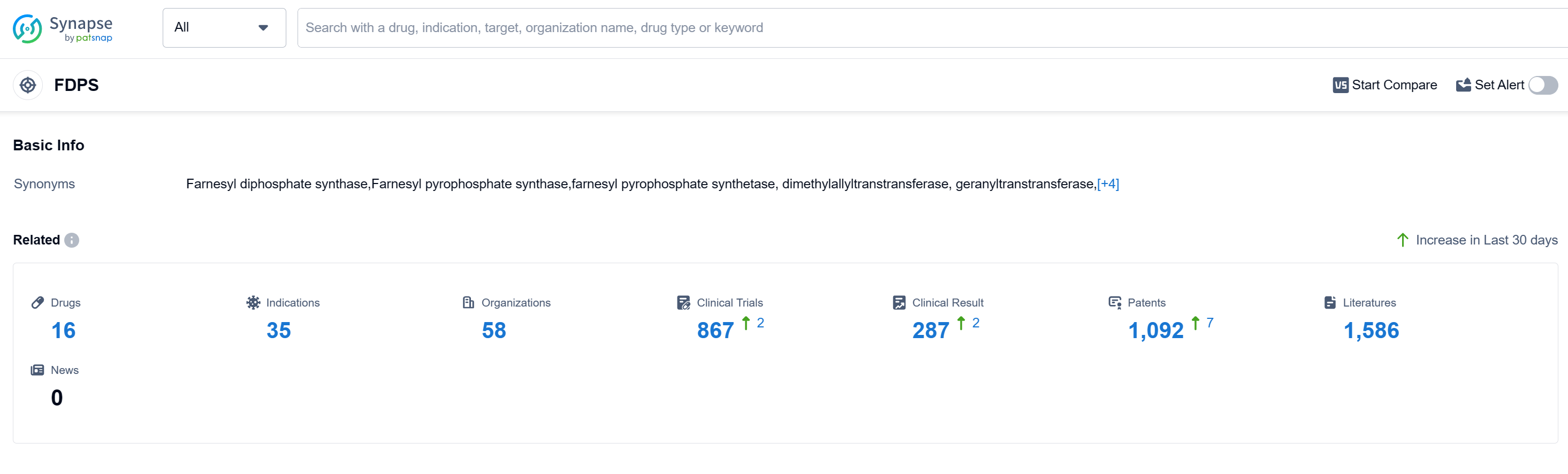
According to Patsnap Synapse, as of 12 Sep 2023, there are a total of 16 FDPS drugs worldwide, from 58 organizations, covering 35 indications, and conducting 867 clinical trials.
Based on the analysis of the data provided, the current competitive landscape of the target FDPS shows that companies like Novartis AG, Teva Pharmaceutical Industries Ltd., Astellas Pharma, Inc., Intas Pharmaceuticals Ltd., and AbbVie, Inc. are growing rapidly with multiple drugs in the approved phase. The most common indication for drugs under the target FDPS is Osteoporosis, followed by other indications such as Hypercalcemia, Osteitis Deformans, and Humoral Hypercalcemia of Malignancy. Small molecule drugs are progressing rapidly, indicating intense competition in the development of innovative drugs. The European Union, Japan, and China are the leading countries/locations in the development of drugs under the target FDPS, with significant progress seen in China. Overall, the target FDPS presents opportunities for growth and development in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Risedronate Sodium is a small molecule drug developed by Procter & Gamble Pharmaceuticals, Inc. It is approved for the treatment of various conditions related to endocrinology and metabolic diseases, as well as skin and musculoskeletal diseases. The drug's primary indication is osteoporosis, including glucocorticoid-induced osteoporosis, osteitis deformans, and postmenopausal osteoporosis. Risedronate Sodium received its first approval in the United States in 1998 and has since been approved in multiple countries globally. It is classified as an orphan drug, indicating its intended use for rare diseases or conditions.