Rezolute starts key Phase 3 trial for RZ358 to treat Congenital Hyperinsulinism
Rezolute, Inc., an innovative enterprise operating in the clinical-phase and concentrating on the creation of breakthrough treatments for severe metabolic disorders and uncommon diseases, has proclaimed the commencement of a critical Phase 3 clinical trial named sunRIZE. This significant research will evaluate the efficacy of RZ358 in individuals diagnosed with congenital hyperinsulinism.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Following the necessary approvals from local authorities and institutional review boards, the inaugural international clinical site beyond American borders has recently initiated operations. This paves the way for the identification and addition of participants. The plan includes progressively bringing more locations online during the upcoming weeks, extending into early 2024.
The company's entrance into the pivotal Phase 3 clinical trial is built on the heels of a successful Phase 2 trial. The earlier study showcased hopeful outcomes in managing congenital hyperinsulinism (cHI), a severe ailment that heavily impacts affected individuals and their loved ones, and for which there is a significant gap in available treatments. The commencement of Phase 3 trials aligns with the company's reception of a priority medicines tag from the European Medicines Agency for RZ358 in October 2023, specifically for cHI management.
"The inauguration of the Phase 3 study signifies an important leap forward in the development journey of RZ358 and underscores the comprehensive progression of our Company," said Nevan Charles Elam, CEO and Founder of Rezolute. He noted the critical need for effective treatments for cHI, citing the limited efficacy and potential adverse effects of the current standard treatment, diazoxide.
Elam expressed his company’s commitment: "There's an acute need for improved treatments. We are eager to collaborate with various global centers to expedite the patient recruitment and screening process for this crucial trial, with the objective of edging nearer to the fulfillment of this treatment goal."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
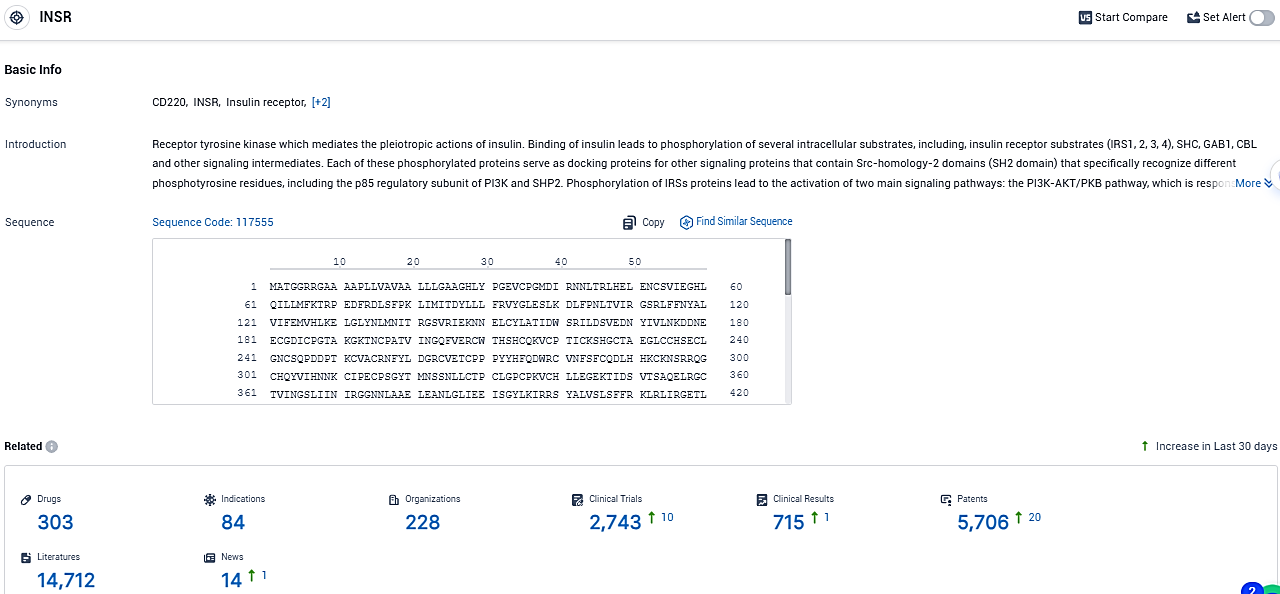
According to the data provided by the Synapse Database, As of December 23, 2023, there are 303 investigational drugs for the INSR target, including 84 indications, 228 R&D institutions involved, with related clinical trials reaching 2743, and as many as 5706 patents.
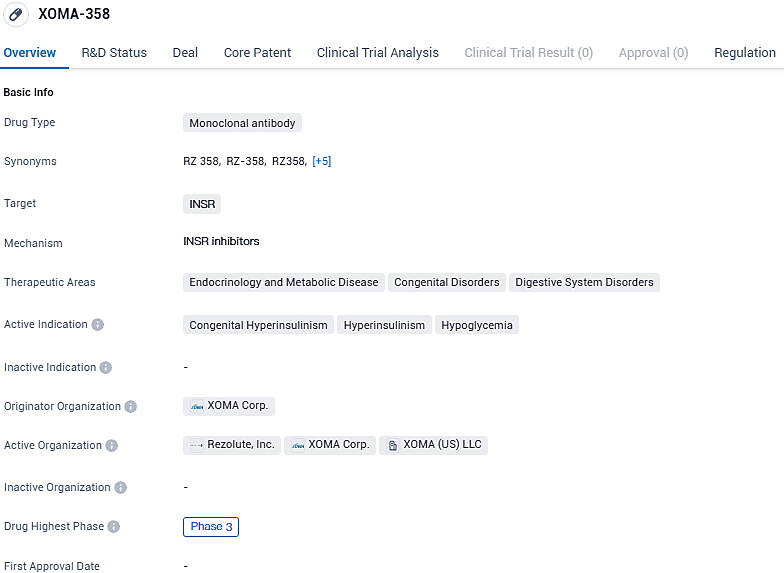
RZ358 shows promise as a potential treatment for congenital hyperinsulinism, hyperinsulinism, and hypoglycemia. Its monoclonal antibody nature and targeting of the insulin receptor make it a unique and innovative approach in the field of biomedicine. RZ358 received Orphan Drug Designation in the United States and European Union for the treatment of congenital HI, as well as Pediatric Rare Disease Designation in the US.