Deciphering SGLT2 Inhibitors and Keeping Up with Their Recent Developments
SGLT2, or sodium-glucose co-transporter 2, is a protein found in the kidneys that plays a crucial role in glucose reabsorption. It is responsible for the reabsorption of glucose from the urine back into the bloodstream. By inhibiting SGLT2, glucose excretion is increased, leading to lower blood glucose levels. This mechanism is particularly important in individuals with type 2 diabetes, as it helps to reduce hyperglycemia. SGLT2 inhibitors are a class of medications that target this protein, providing an effective treatment option for managing diabetes and improving glycemic control.
SGLT2 inhibitors mainly work by inhibiting SGLT2 in renal tubules, reducing the renal glucose threshold, reducing the reabsorption of glucose by renal tubules, increasing the excretion of glucose in urine, and thereby lowering plasma glucose levels. With the conduct of clinical research, the hypoglycemic efficacy and safety of SGLT2 inhibitors have been fully confirmed in global clinical studies. Cardiovascular outcome studies of SGLT2 inhibitors have confirmed that they can significantly reduce the risk of cardiovascular events in patients with type 2 diabetes. Among them, the DECLARE-TIMI58 study showed that dapagliflozin reduced the risk of heart failure hospitalization or cardiovascular (CV) death by 17% (p=0.005), and did not increase the risk of major adverse cardiovascular events (MACE) (p=0.17); other studies have shown the benefits of SGLT2 inhibitors in delaying the progression of end-stage renal disease: dapagliflozin can delay the time for CKD patients to enter end-stage renal disease by 6.6 years. In addition, in terms of diabetes-related complications, exploratory studies of SGLT2 inhibitors have also achieved results. At this year’s American Diabetes Association (ADA) annual meeting, two studies from Japan showed that SGLT2 inhibitors can improve diabetic neuropathy and impaired oxidative muscle function in patients with type 2 diabetes. The progress of evidence promotes the update of the guidelines. The 2023 latest version of ADA diabetes clearly points out that for patients with type 2 diabetes with high cardiovascular and renal risk, drugs such as SGLT2 inhibitors need to be part of the strategy to lower blood sugar and comprehensively reduce cardiovascular and renal risk, and can be independent of HbA1c targets. The status of SGLT2 inhibitors in the treatment of type 2 diabetes has been further improved.
The analysis of the target SGLT2 reveals a competitive landscape with multiple companies actively involved in the development of drugs. C.H. Boehringer Sohn AG & Co. KG, Merck & Co., Inc., AstraZeneca PLC, Daewoong Co., Ltd., and Johnson & Johnson are the companies growing fastest under the target SGLT2. The most common indication for approved drugs is Diabetes Mellitus, Type 2. Small molecule drugs dominate the drug types progressing rapidly under the target SGLT2. The United States, European Union, and China are the countries/locations with the highest development progress. The target SGLT2 shows promising future development with ongoing research and development efforts by various companies and countries/locations.
How do they work?
SGLT2 inhibitors are a type of medication used in the treatment of type 2 diabetes mellitus. SGLT2 stands for sodium-glucose co-transporter 2, which is a protein responsible for reabsorbing glucose in the kidneys. By inhibiting this protein, SGLT2 inhibitors help to lower blood glucose levels by increasing the amount of glucose excreted in the urine.
From a biomedical perspective, SGLT2 inhibitors work by blocking the reabsorption of glucose in the kidneys, allowing excess glucose to be eliminated from the body. This mechanism of action helps to reduce blood glucose levels and improve glycemic control in patients with type 2 diabetes.
It is important to note that SGLT2 inhibitors are not suitable for everyone and should be used under the guidance of a healthcare professional. They have been shown to have additional benefits beyond glycemic control, such as promoting weight loss and reducing the risk of cardiovascular events. However, they may also have side effects, including an increased risk of urinary tract infections and genital mycotic infections. Therefore, it is crucial for patients to discuss the potential risks and benefits of SGLT2 inhibitors with their healthcare provider before starting this medication.
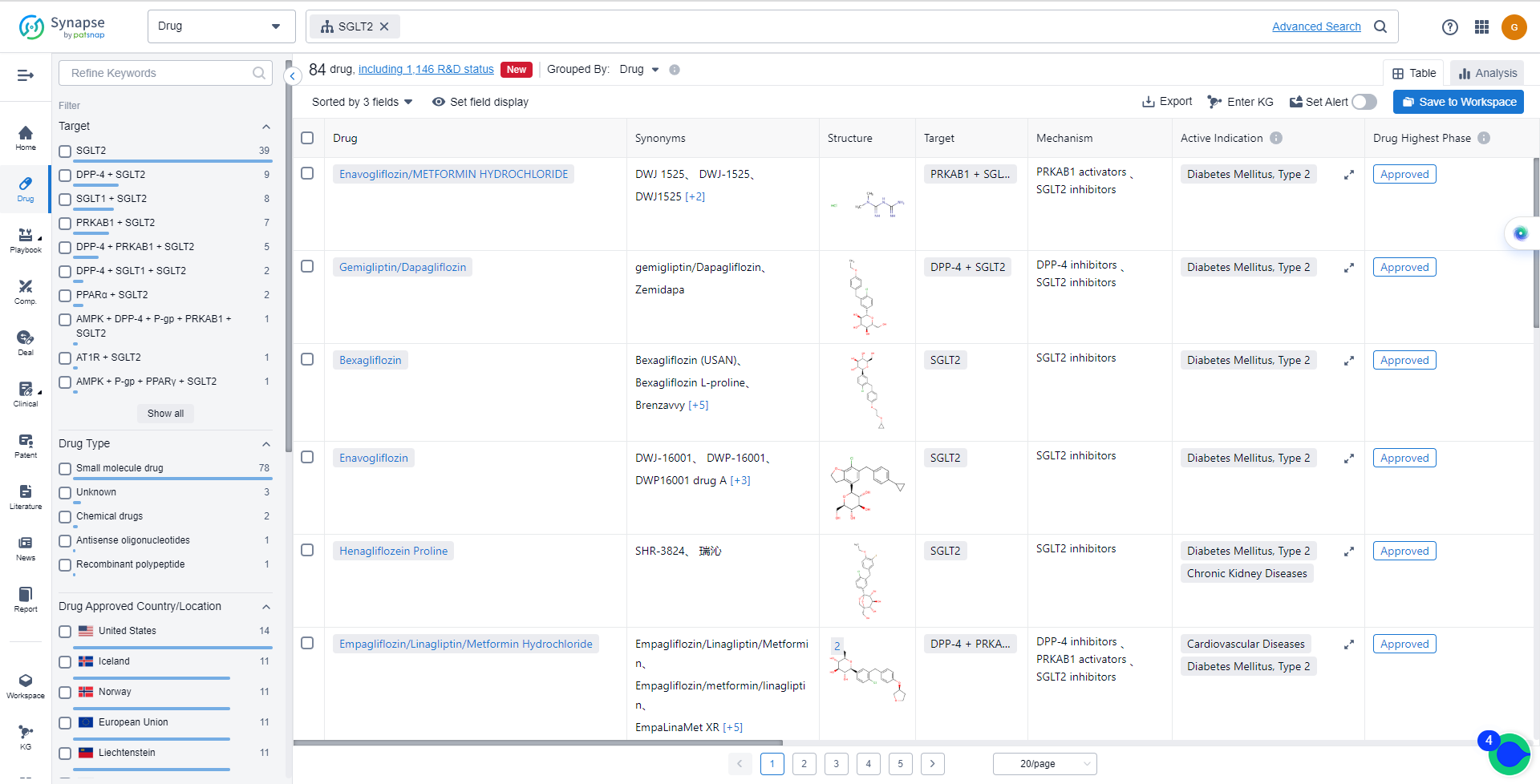
List of SGLT2 Inhibitors
The currently marketed SGLT2 inhibitors include:
- Enavogliflozin/METFORMIN HYDROCHLORIDE
- Gemigliptin/Dapagliflozin
- Bexagliflozin
- Enavogliflozin
- Henagliflozein Proline
- Empagliflozin/Linagliptin/Metformin Hydrochloride
- Metformin/Remogliflozin etabonate
- Remogliflozin etabonate
- Sotagliflozin
- Ertugliflozin
For more information, please click on the image below.
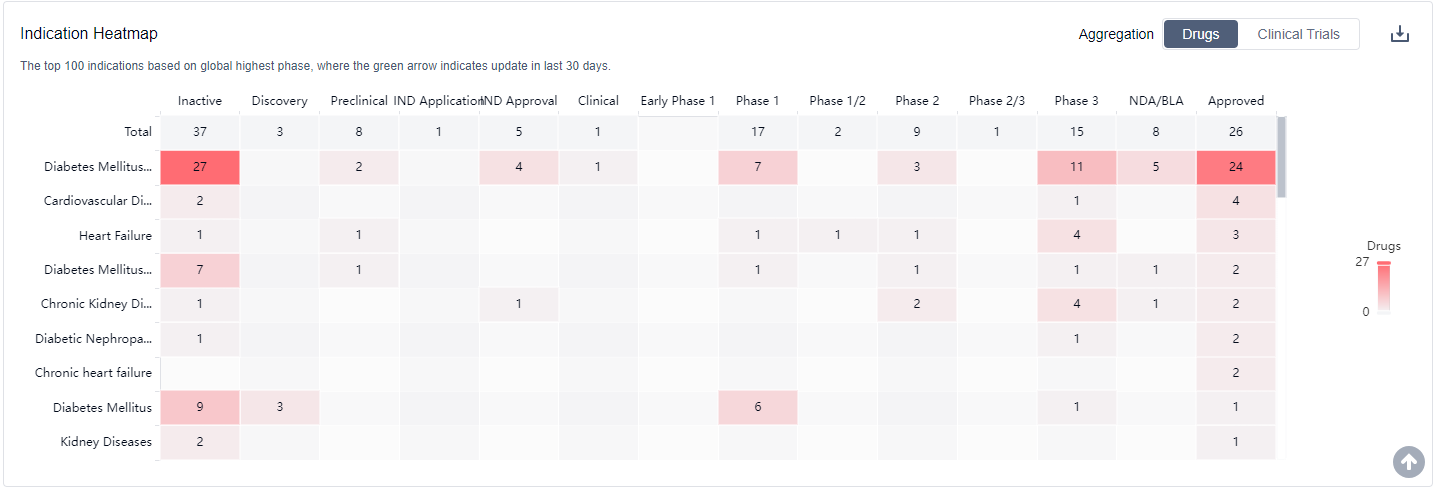
What are SGLT2 inhibitors used for?
SGLT2 inhibitors have been studied for their potential in treating various conditions. They are particularly promising in the following areas Type 2 Diabetes Mellitus;Chronic Kidney Disease;Heart Failure. For more information, please click on the image below to log in and search.
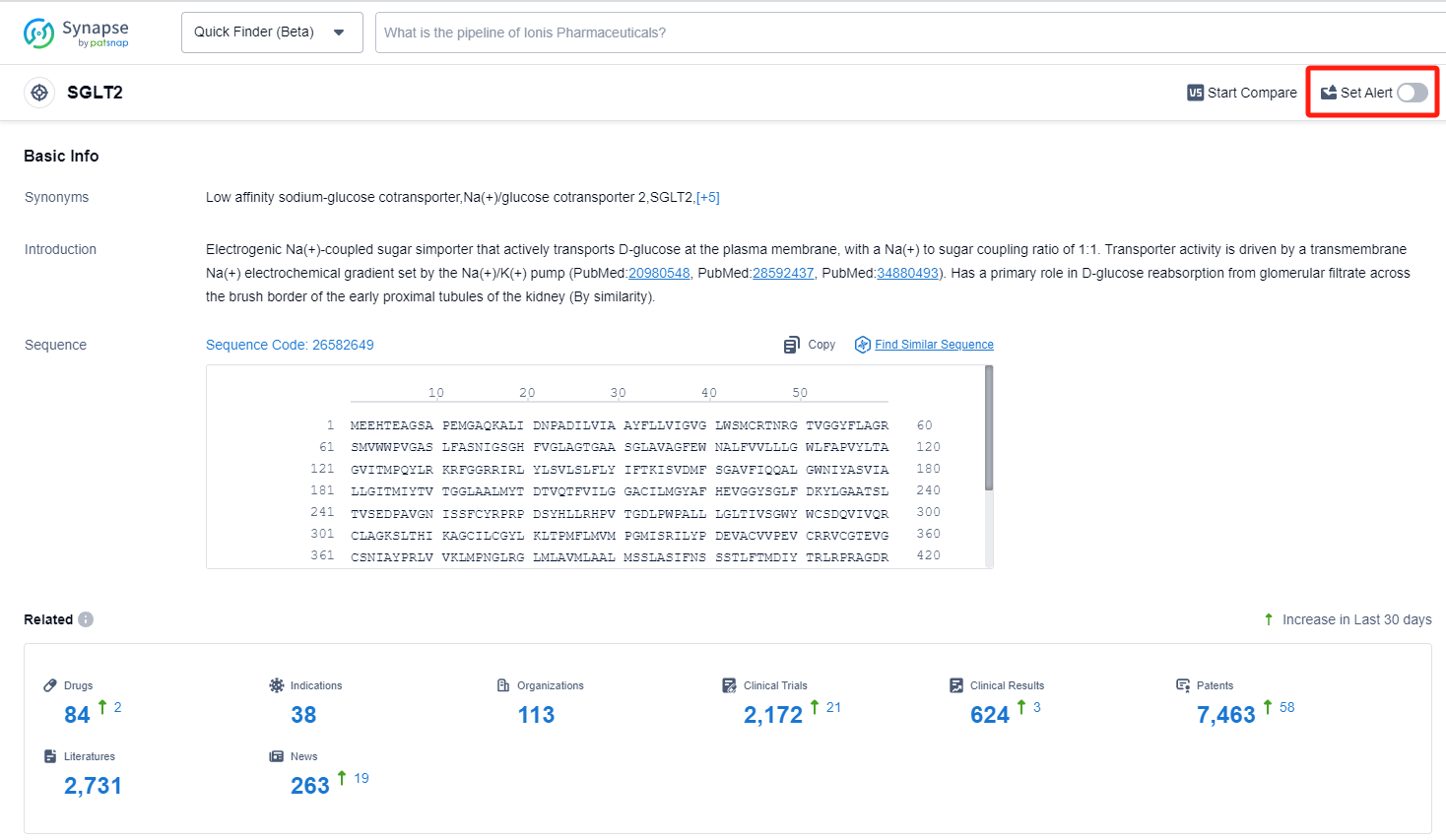
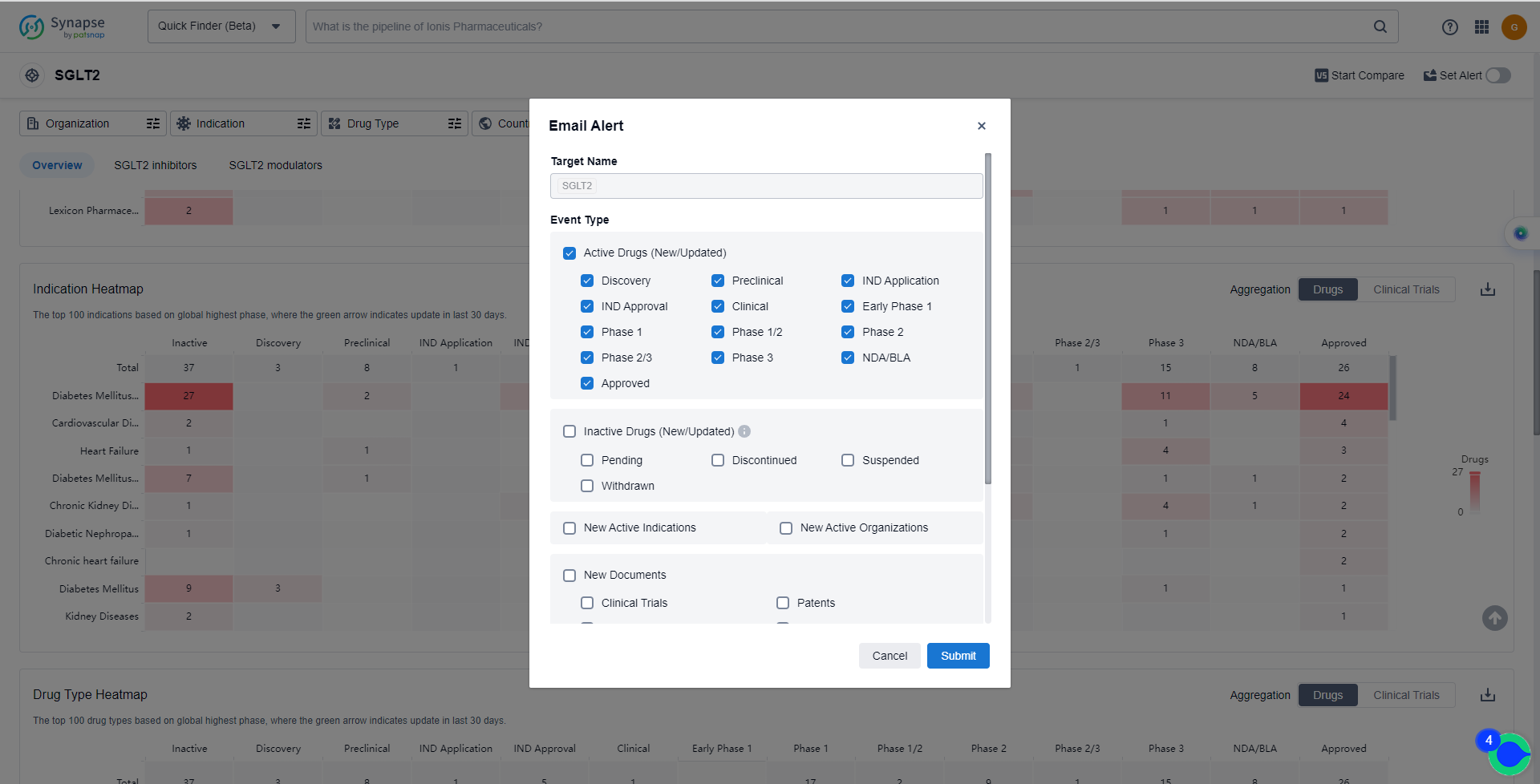
How to obtain the latest development progress of SGLT2 inhibitors?
In the Synapse database, you can keep abreast of the latest research and development advances of SGLT2 inhibitors anywhere and anytime, daily or weekly, through the "Set Alert" function. Click on the image below to embark on a brand new journey of drug discovery!