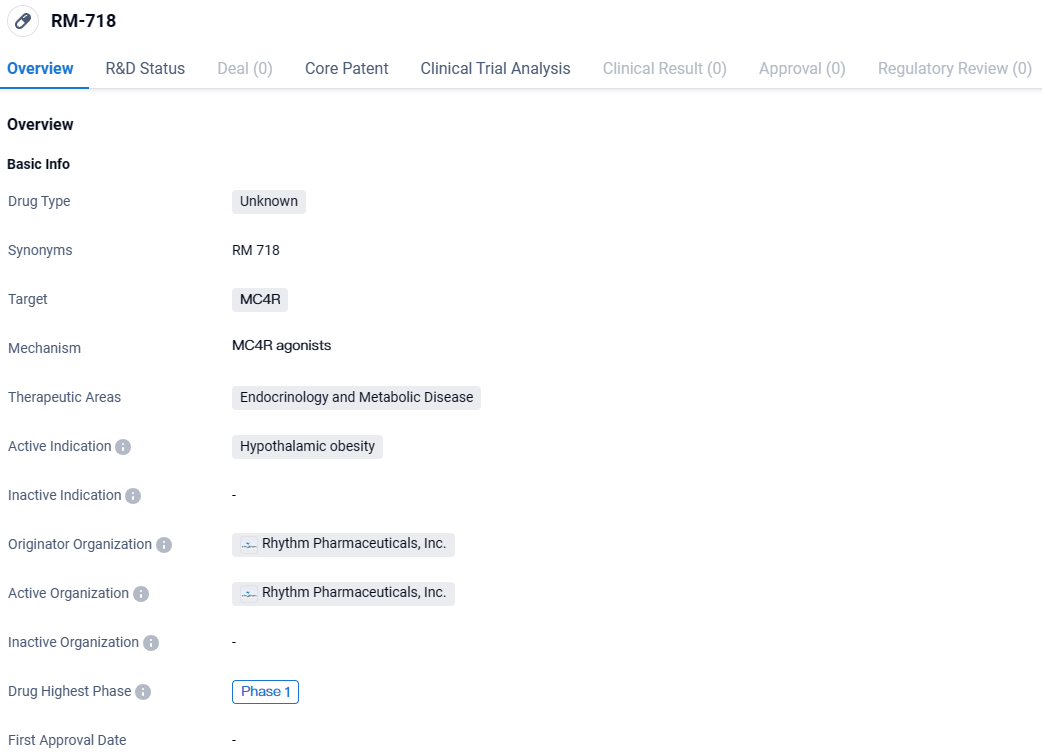
Rhythm Pharma Begins Human Trials for Weekly MC4R-Targeting Drug RM-718
Rhythm Pharmaceuticals, Inc., an enterprise engaged in the development and marketing of drugs for rare neuroendocrine disorders, has reported the initiation of treatment with the investigational drug RM-718 in the earliest participants of their Phase 1 clinical study. RM-718 is a novel, once-weekly therapeutic candidate that selectively activates the melanocortin-4 receptor (MC4R) while intentionally avoiding activity on the MC1R receptor, in hopes of preventing any associated increase in skin pigmentation.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
We are thrilled to launch the clinical trials for RM-718, our innovative MC4R agonist therapy. With promising initial findings from our laboratory studies, we've recognized that RM-718 may have the capacity to mitigate hyperphagia—an excessive eating disorder leading to irregular seeking of food—as well as address the profound issue of obesity, which are commonly observed in the unique disorders associated with the MC4R pathway," proclaimed David Meeker, M.D., the chief authority, President, and CEO at Rhythm.
Dr. Meeker also stated, "Furthermore, RM-718 is engineered to permit once-weekly administration and is intended to minimize the risk of hyperpigmentation."
The initial phase clinical trial is divided into a trio of segments to determine its safety, tolerability, and pharmacokinetic properties. The trial includes a Phase A, where single increasing doses of RM-718 will be given to healthy individuals between 18 to 55 who are obese; a Phase B, involving multiple increasing doses of RM-718 administered to same demographic; and finally, a Phase C: multiple ascending doses to patients aged 12 to 65 with hypothalamic obesity. Both Phase A and B groups will be part of a double-blind, placebo-controlled study with a 2:1 randomization.
During the trial, participants in Phase A will be given a solitary weekly administration of RM-718 or a placebo. In contrast, those in Phase B will receive this treatment or placebo across four weeks, and all subjects in Phase C will be given open-label RM-718 across four weeks. RM-718, or its placebo equivalent, will be delivered through a subcutaneous injection once every week. The trial aims to include a total of 96 participants.
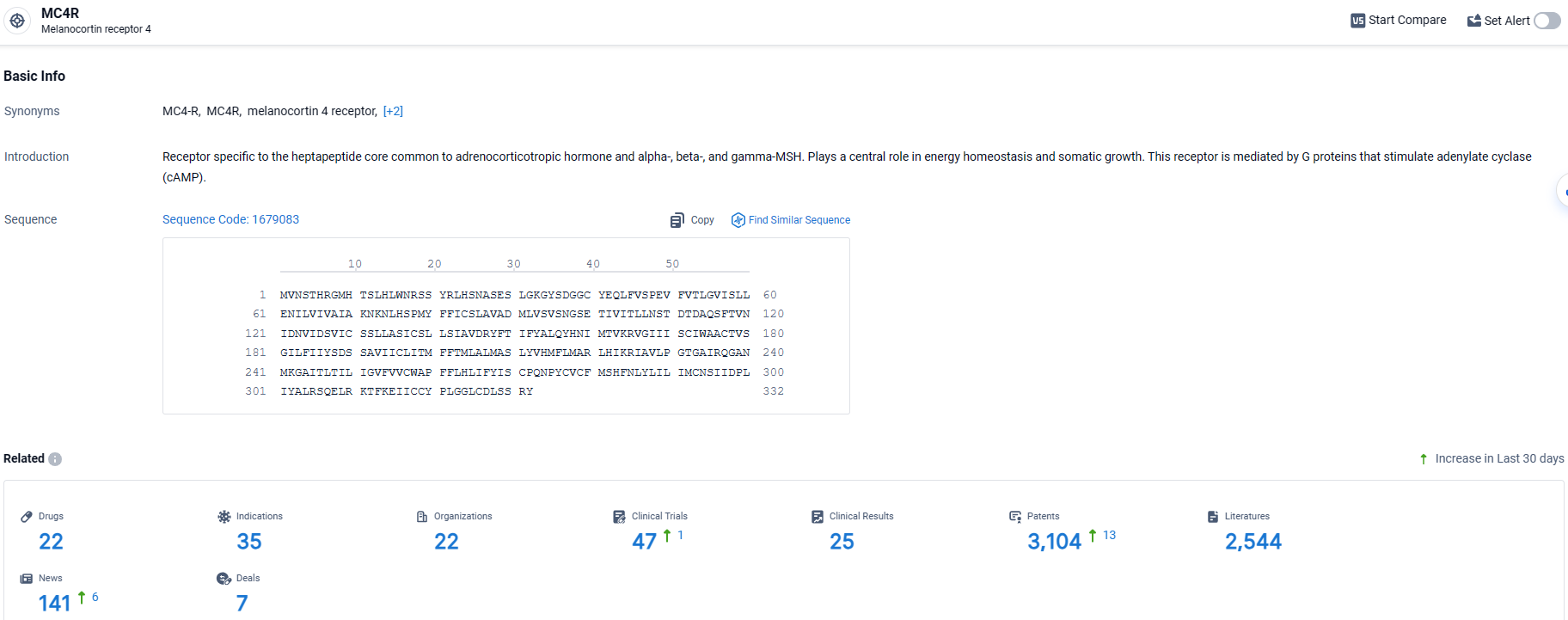
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of March 27, 2024, there are 22 investigational drugs for the MC4R target, including 35 indications, 22 R&D institutions involved, with related clinical trials reaching 47, and as many as 3104 patents.
RM-718 is an investigational, weekly, MC4R-specific agonist that has demonstrated the potential to reduce body weight and hunger in preclinical studies. RM-718 is designed to be highly selective and MC1Rsparing and thereby not cause hyperpigmentation. The drug has reached Phase 1 of development, indicating progress in its clinical testing.