Rila Therapeutics Starts Phase 1 Trials of RLA-23174 for Chronic Kidney Disease and Fibrosis
Rila Therapeutics, Inc., a biotechnology firm dedicated to combating chronic fibrotic diseases, revealed that the initial group of participants received doses in a phase 1 study of RLA-23174 in China, in partnership with their development collaborator, Yingli Pharmaceutical. RLA-23174 represents a pioneering small molecule allosteric inhibitor of HIPK2, which obstructs TGF-β signaling to tackle FSGS and other fibrotic conditions.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The initial phase 1 placebo-controlled trial for RLA-23174 aims to evaluate its safety across various dose levels, its tolerability, the impact of food intake, and its pharmacokinetic profile. This single-site study is recruiting healthy volunteers and includes staggered cohorts for single ascending doses and multiple ascending doses. The study plans to enroll up to 80 participants, with completion anticipated by the end of Q3 2024, to facilitate progression into the Phase 2 clinical program.
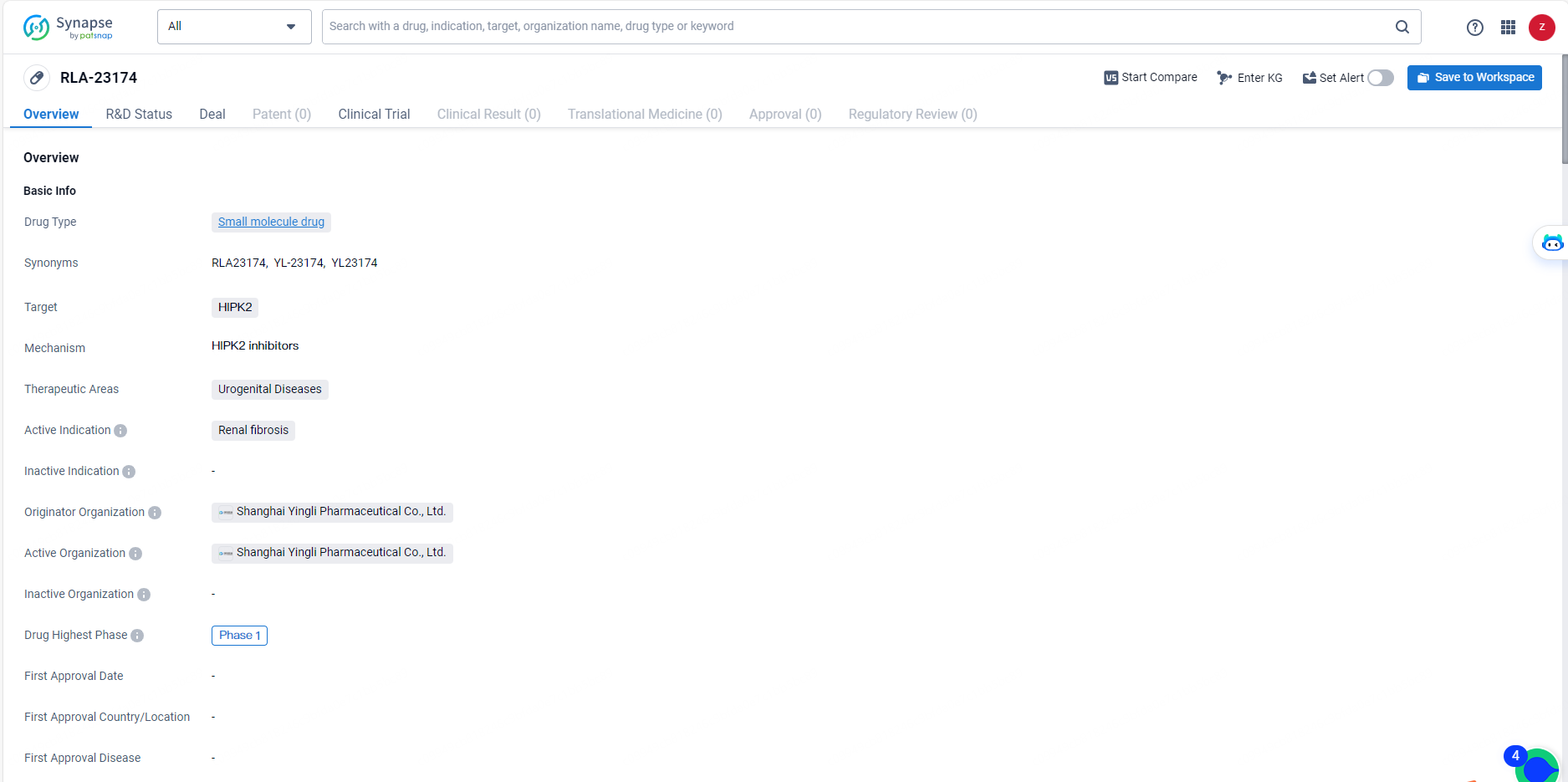
RLA-23174 is an innovative small molecule allosteric inhibitor of HIPK2, potentially representing a first-in-class therapeutic option for pan-organ fibrosis. It works by disrupting the TGF-β/Smad3 signaling pathway through modulating the interaction between HIPK2 and Smad3, without hindering the kinase activities of HIPK2 or the TGF-β receptors.
Thus, RLA-23174 exhibits anti-fibrotic properties by mitigating TGF-β signaling without inducing severe adverse effects associated with total HIPK2 or TGF-β pathway inhibition. Preclinical research indicates that RLA-23174 possesses strong anti-fibrotic efficacy, notable in vivo activity, excellent pharmacokinetic traits in animal models, and a favorable safety profile. The compound shows promising drug-like features and substantial developmental potential.
"The administration of the first cohort in this initial human trial for RLA-23174 marks a significant milestone for Rila Therapeutics. It highlights our transition into a clinical development organization, drawing us nearer to benefiting patients with kidney disease," remarked Robert Drakas, Ph.D., CEO of Rila Therapeutics.
"There is a pressing need to develop more effective treatments to prevent disease progression, and we firmly believe that anti-fibrosis medication represents a promising avenue for such intervention. We are optimistic that HIPK2 inhibitors like RLA-23174 could offer additional renal protection alongside current CKD therapies," commented John He, MD, PhD, founder and Chief of Nephrology at Icahn Mount Sinai, a leading expert at one of the largest medical institutions in the United States.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
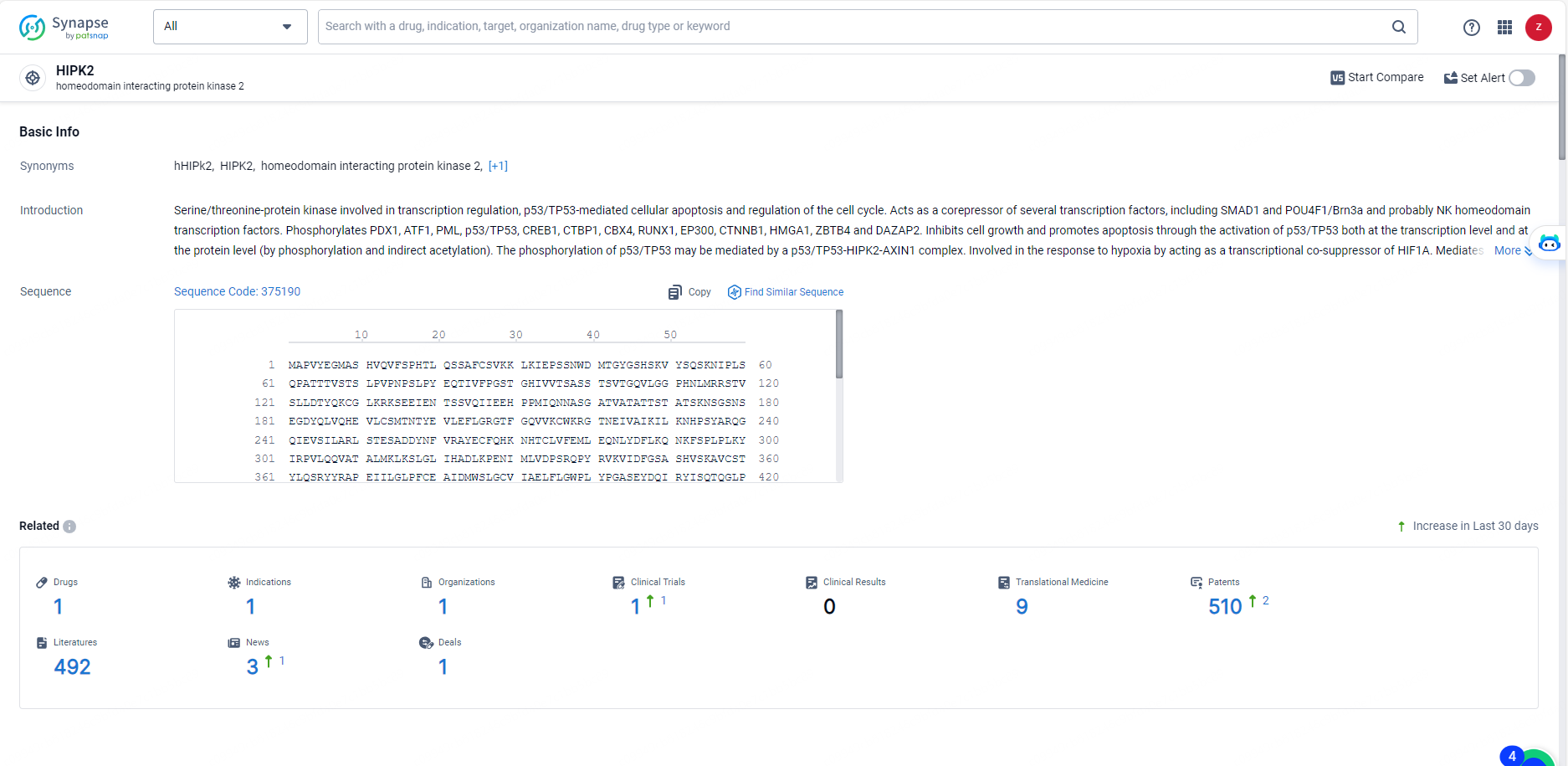
According to the data provided by the Synapse Database, As of May 27, 2024, there are 1 investigational drugs for the HIPK2 targets, including 1 indications, 1 R&D institutions involved, with related clinical trials reaching 1, and as many as 510 patents.
RLA-23174 is a small molecule drug targeting HIPK2, with a focus on treating renal fibrosis within the therapeutic area of urogenital diseases. With its current status at Phase 1 of clinical development, the drug holds potential for addressing an unmet medical need in the field of biomedicine. Further advancements in its development and potential partnerships may contribute to the eventual availability of RLA-23174 as a treatment option for patients with renal fibrosis.