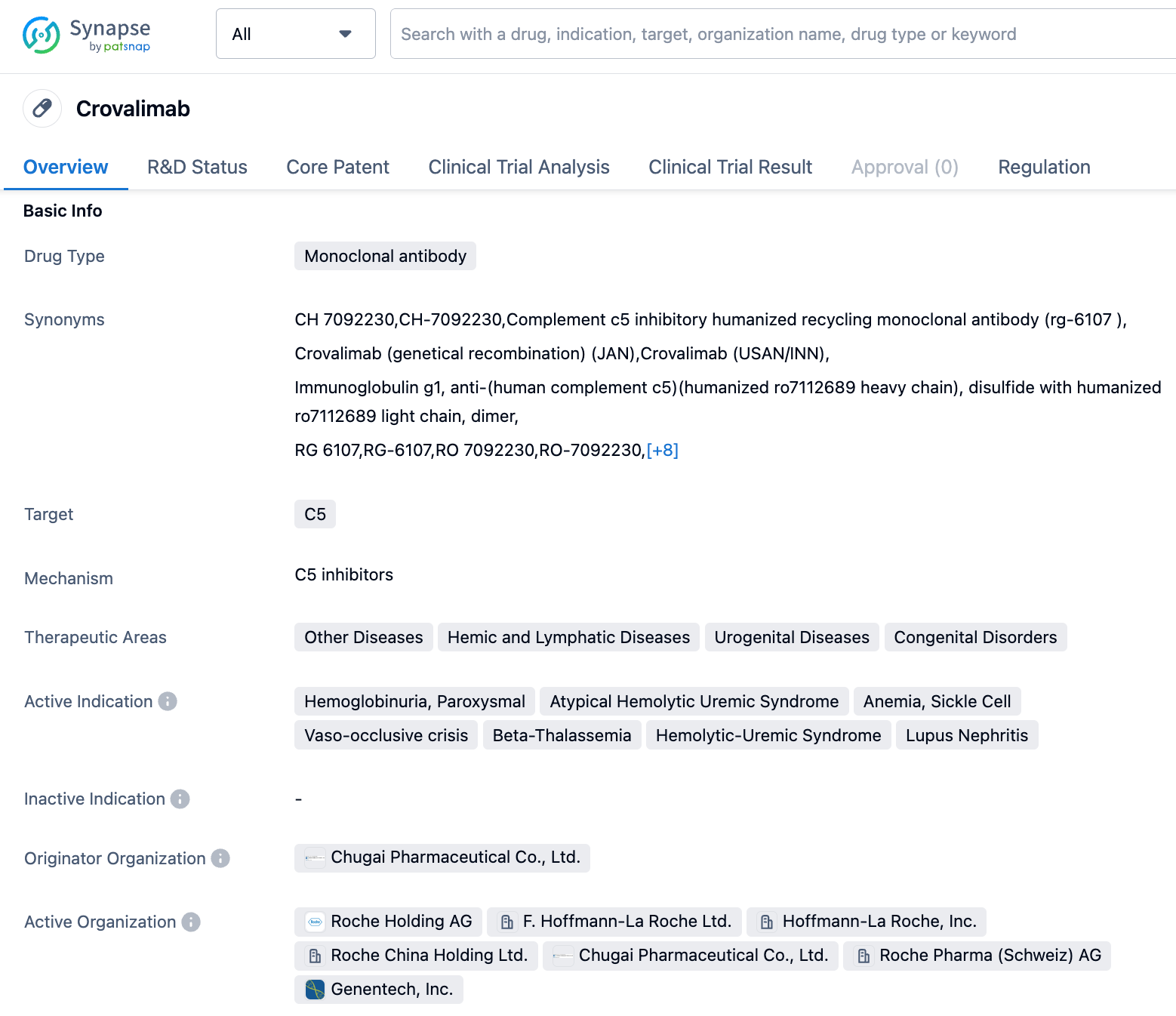
Roche's new generation of C5 complement monoclonal antibody, crovalimab, applies for market approval in the US
On September 6, Genentech, a member of the Roche, announced that the U.S. FDA has accepted the company's Biologics License Application (BLA) for the novel complement C5 monoclonal antibody Crovalimab for the treatment of paroxysmal nocturnal hemoglobinuria (PNH). If approved, Crovalimab will be the first once-monthly subcutaneous formulation for the treatment of PNH that patients can self-administer.
Crovalimab is a new generation C5 inhibitor Roche developed which has been engineered through continuous monoclonal antibody recovery technology (Smart-Ig). It can block the complement C5 from breaking down into C5a and C5b, with the potential to inhibit complement activation. The drug combines isoelectric point, neonatal Fc receptor (FcRn), and pH-dependent affinity technologies, which not only allow it to bind efficiently to C5, but also enhances endothelial cell absorption of Crovalimab binding to C5. It also enables efficient FcRn-mediated re-circulation of crovalimab. In August 2022, the application for the domestic listing of Crovalimab injection was accepted and included in the priority review varieties, intended for the treatment of PNH. Beyond PNH, Crovalimab is currently under development for atypical hemolytic uremic syndrome (aHUS), sickle cell disease, and other complement-mediated diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
This filing was enabled by encouraging results from two Phase III studies (COMMODORE 1 and COMMODORE 2). COMMODORE 1 is a multicenter, randomized, open-label, active-controlled Phase III clinical trial that evaluated the safety of switching to Crovalimab from Eculizumab or Ravulizumab. COMMODORE 2 is a multicenter, randomized, open-label, active-controlled Phase III clinical trial that evaluated the efficacy and safety of Crovalimab (administered subcutaneously every 4 weeks) compared to Eculizumab (administered intravenously every 2 weeks) in PNH patients who have not previously received complement inhibitor therapy. The results showed a risk-reduced benefit for patients switching to Crovalimab from Eculizumab or Ravulizumab in the COMMODORE 1 study, and non-inferiority of Crovalimab to Eculizumab in the COMMODORE 2 study.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
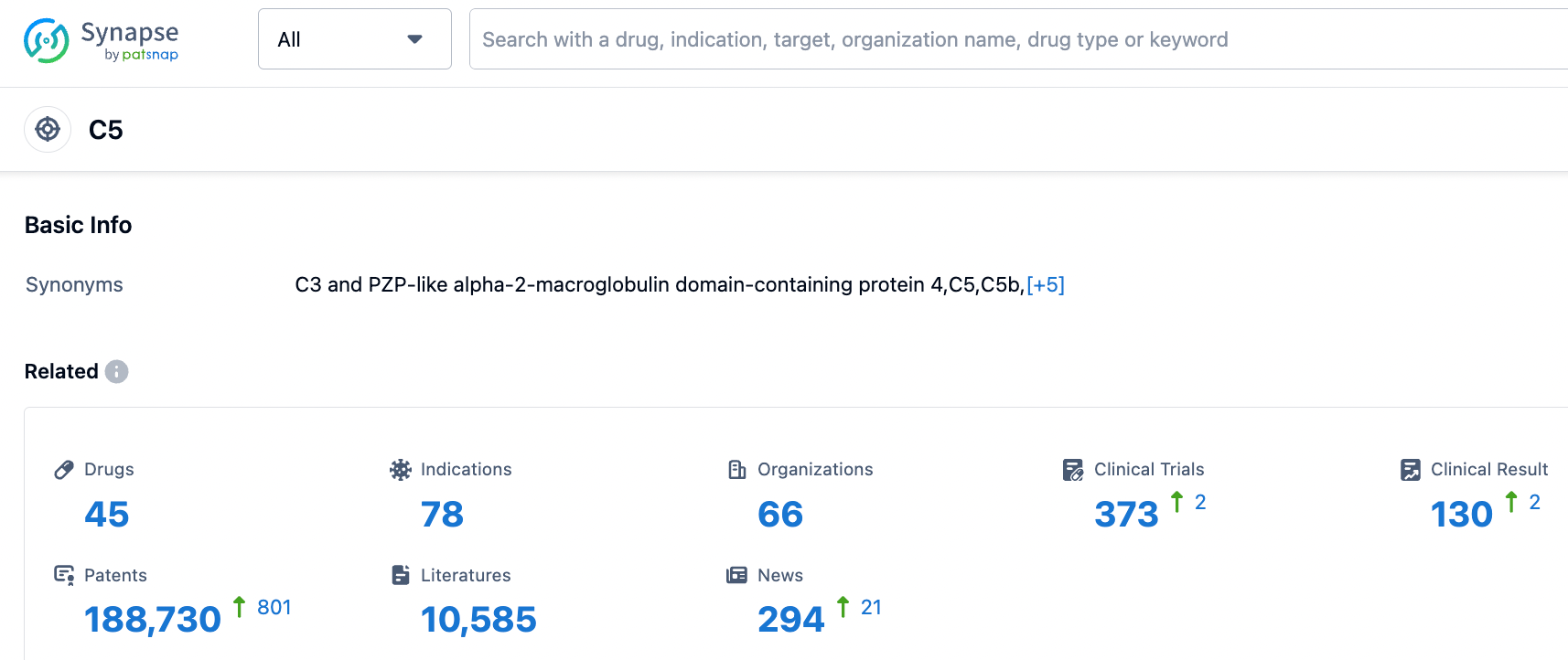
According to the information disclosed by the Synapse database, as of September 7, 2023, there were 45 drugs under investigation for the C5 target, with 78 applicable diseases, 66 research institutions involved, 373 related clinical trials, and as many as 188,298 patents... The complement protein C5 is a very successful drug target. The first drug, Soliris (Eculizumab), had sales of $4.064 billion in 2020 and $3.762 billion in 2022. Crovalimab, as a subcutaneous formulation that only needs to be administered once a month and can be self-administered by patients, greatly facilitates its use and has the potential to become a blockbuster drug.