Sage Therapeutics Ends Partnership with Biogen on SAGE-324 Project
Sage Therapeutics, Inc. (NASDAQ: Sage) disclosed that Biogen has relinquished its rights under the collaboration and license deal with Sage concerning the SAGE-324 program. The two firms recently reported unfavorable outcomes from the Phase 2 KINETIC 2 Study of the experimental SAGE-324 for the ongoing treatment of essential tremor (ET) and have halted further clinical progression of SAGE-324 in ET.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Sage and Biogen will maintain their collaboration on ZURZUVAE® (zuranolone), the pioneering oral medication approved by the FDA for the treatment of postpartum depression (PPD) in women. They will further pursue their mutual goal of supporting women affected by PPD.
According to the collaboration and license agreement, the termination will take effect on February 17, 2025, at which point Sage will regain full ownership of the SAGE-324 asset. Sage intends to continue investigating other potential uses for SAGE-324.
ZURZUVAE (zuranolone) CIV functions as a neuroactive steroid GABAA receptor positive modulator and is prescribed for treating postpartum depression in adults.
This overview doesn't contain all necessary details for using ZURZUVAE safely and effectively. Refer to the full prescribing information for ZURZUVAE for comprehensive guidelines.
ZURZUVAE can cause serious side effects. The most frequent side effects include sleepiness, dizziness, the common cold, diarrhea, fatigue, and urinary tract infection. ZURZUVAE might impair your alertness and ability to drive or perform hazardous tasks. Refrain from driving, operating machinery, or engaging in dangerous activities for at least 12 hours after taking a dose during the 14-day treatment period. You may not be aware of your ability to drive safely or the impact of the drug on you.
ZURZUVAE may lead to sleepiness, slow thinking, dizziness, confusion, and difficulty walking, increasing your fall risk during treatment. Consuming alcohol, CNS depressants, or opioids while on ZURZUVAE can worsen these symptoms and may cause breathing difficulties. Inform your healthcare provider if you experience these symptoms or if they worsen. Your provider may reduce your dose or discontinue ZURZUVAE.
ZURZUVAE is a C-IV controlled substance due to the presence of zuranolone, which has abuse potential or could lead to dependence. Notify your healthcare provider immediately if you become pregnant or plan to conceive during treatment. Effective contraception should be used during treatment and for one week after the last dose. ZURZUVAE and other antidepressants might elevate the risk of suicidal thoughts and actions in individuals 24 years or younger. ZURZUVAE is not intended for pediatric use.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
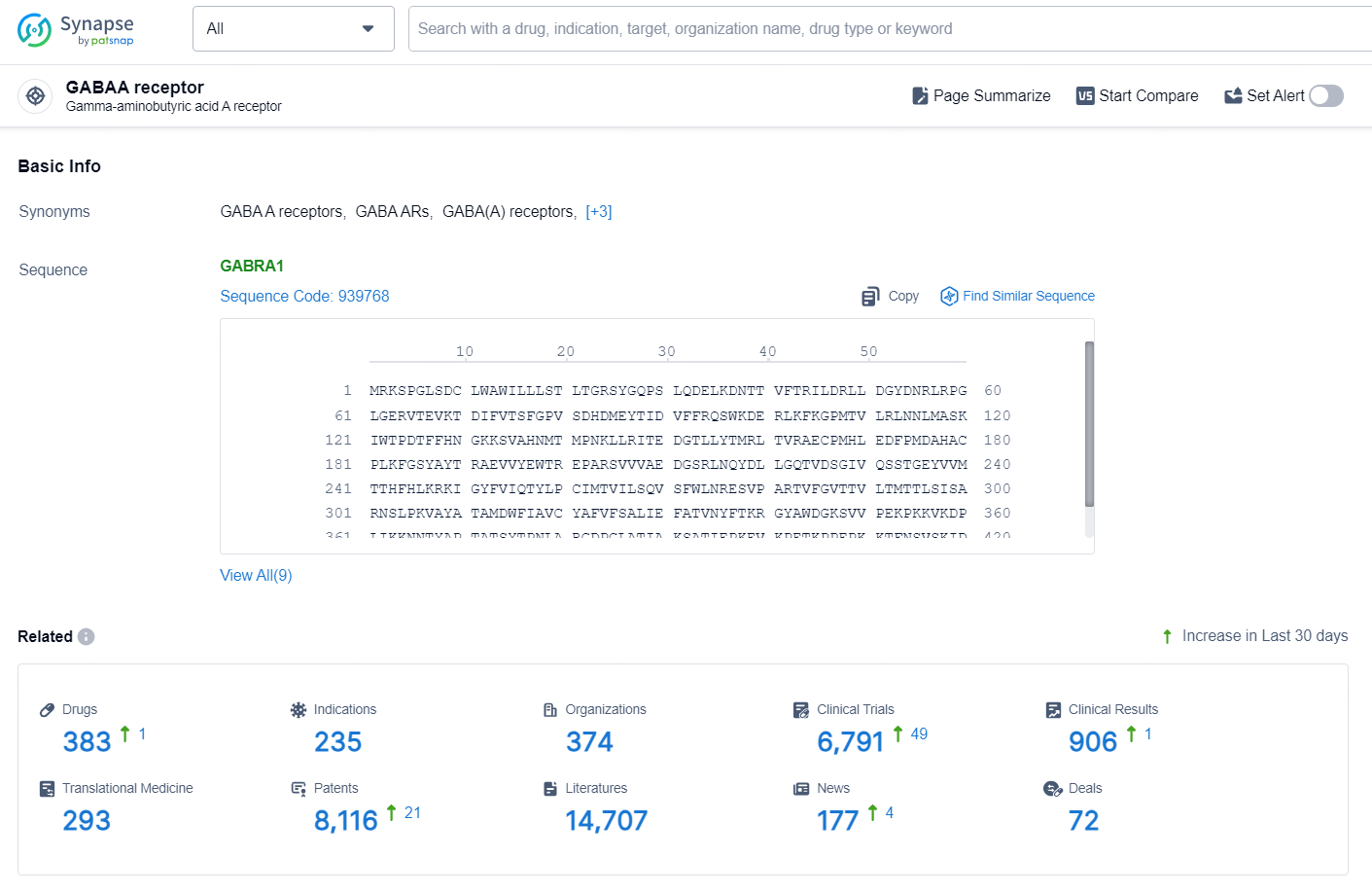
According to the data provided by the Synapse Database, As of September 29, 2024, there are 383 investigational drugs for the GABAA receptor targets, including 235 indications, 374 R&D institutions involved, with related clinical trials reaching 6791, and as many as 8116 patents.
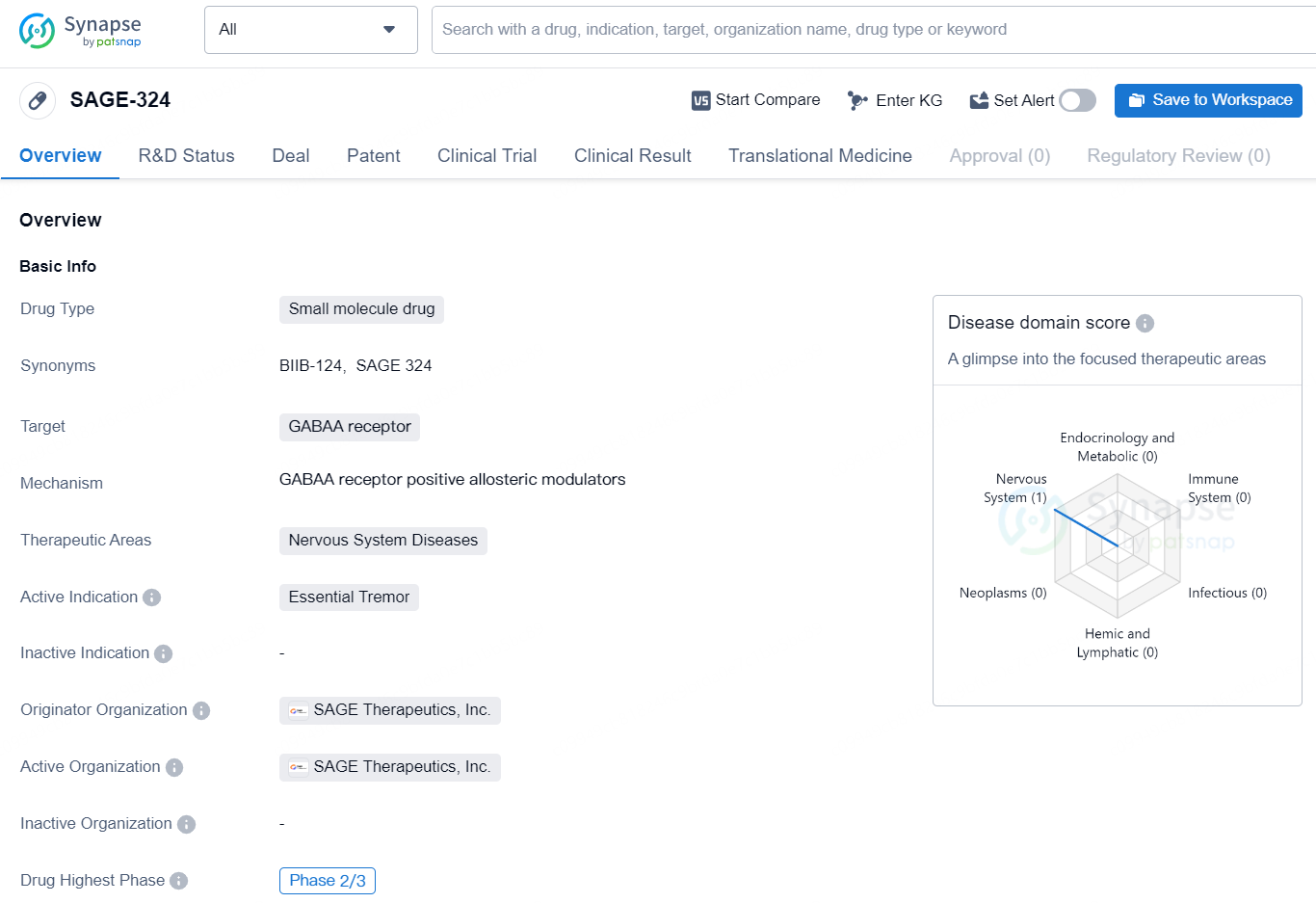
SAGE-324 is a small molecule drug developed by SAGE Therapeutics, Inc. It targets the GABAA receptor and is intended for the treatment of nervous system diseases, specifically essential tremor. The drug is currently in its highest phase of development, which is Phase 2/3. The GABAA receptor is a protein complex that plays a role in the inhibitory neurotransmission within the central nervous system. By targeting this receptor, SAGE-324 aims to modulate the neural signaling associated with essential tremor, a neurological disorder characterized by uncontrollable shaking or trembling, primarily in the hands.