Satellos Reports Initial Participant Dosed in Phase 1 Clinical Trial of SAT-3247
Satellos Bioscience Inc. ("Satellos" or the "Company") (TSX: MSCL) (OTCQB: MSCLF), a publicly traded biotechnology firm focused on the development of innovative small molecule-based therapies for muscle diseases and disorders, announced that the initial participant has received a dose in a Phase 1 clinical trial of SAT-3247. SAT-3247 is an innovative, oral small molecule medication aimed at AAK1, intended to regenerate skeletal muscle tissue in conditions such as Duchenne muscular dystrophy (DMD or Duchenne) as well as other degenerative or muscle-injury related conditions.
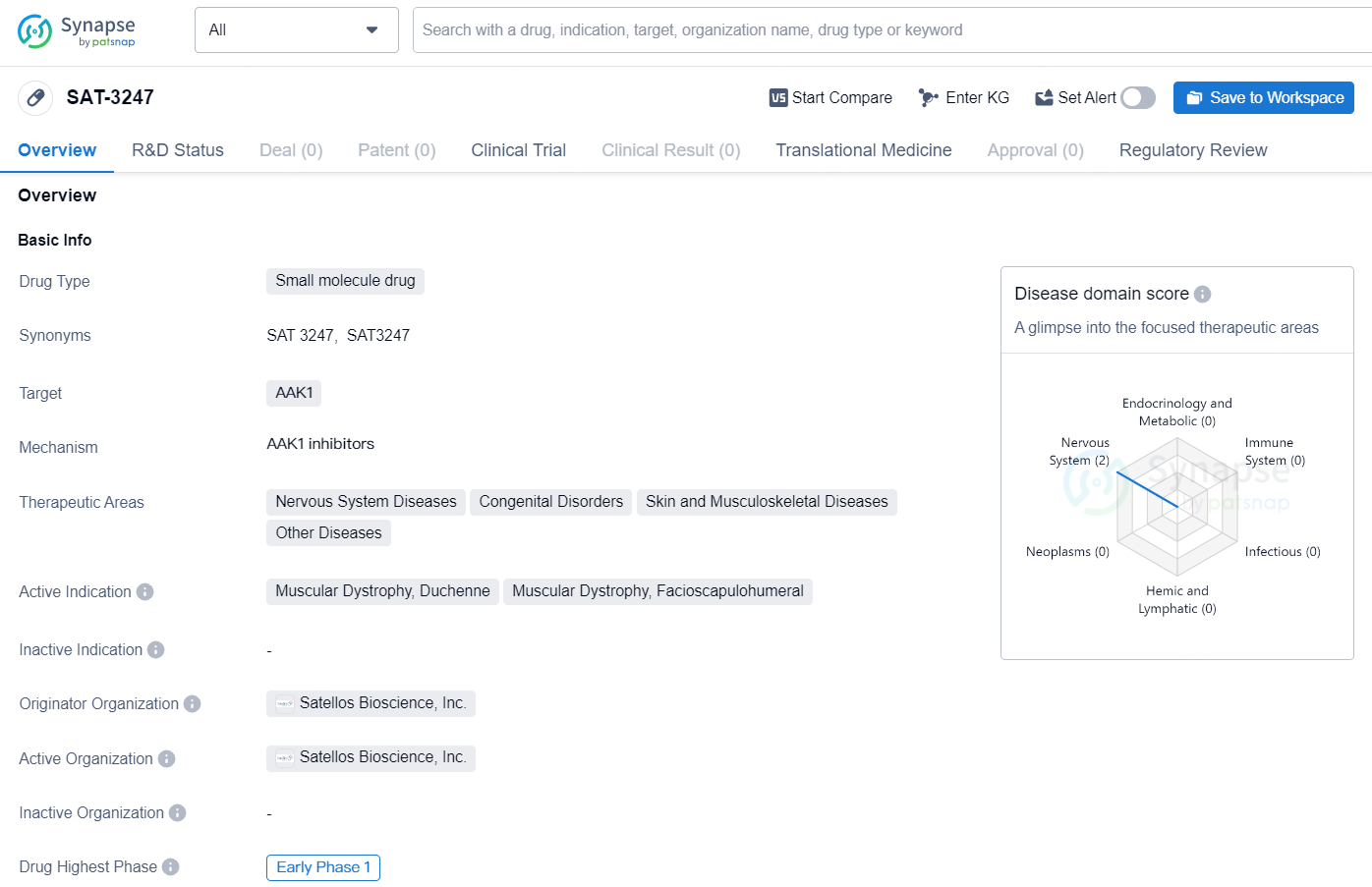
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Frank Gleeson, Co-founder and CEO of Satellos, stated, “We are thrilled to share that we have begun dosing participants in the Phase 1 clinical trial of SAT-3247, marking the transition of Satellos into a clinical-stage company. This is a crucial step in our mission to innovate treatments aimed at muscle regeneration for individuals affected by Duchenne and other muscle degeneration or injury-related conditions. We are eager to move forward with this trial to acquire meaningful data on the safety and pharmacological characteristics of SAT-3247.”
The Phase 1 trial will be divided into two parts. The first part will involve 72 healthy participants in a double-blind, randomized, placebo-controlled, staggered, parallel-group study to evaluate SAT-3247’s safety and pharmacokinetics. Participants will be distributed across five single-ascending dose (SAD) cohorts, four multiple-ascending dose (MAD) cohorts, and one cohort assessing the effect of food on drug absorption. The second part, anticipated to commence in late Q4 2024, will involve 10 adult volunteers with genetically confirmed Duchenne Muscular Dystrophy (DMD) in a 28-day, open-label, single dose cohort. This will examine the drug's safety and pharmacokinetics in patients while probing potential pharmacodynamic markers.
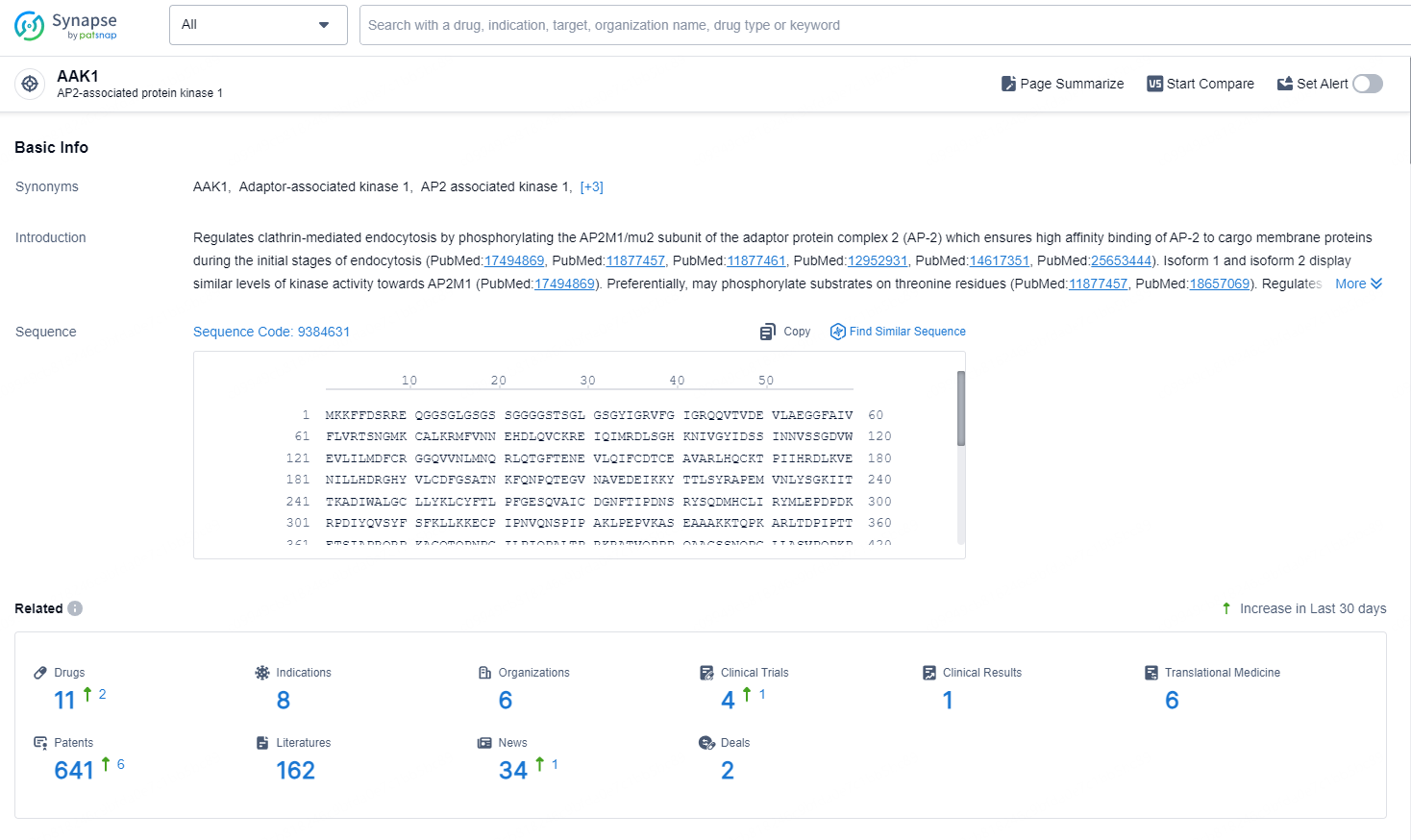
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 18, 2024, there are 11 investigational drug for the AAK1 targets, including 8 indications, 6 R&D institutions involved, with related clinical trials reaching 4, and as many as 641 patents.
SAT-3247 is a small molecule drug developed by Satellos Bioscience, Inc. The drug targets AAK1 and falls within the therapeutic areas of Nervous System Diseases, Congenital Disorders, Skin and Musculoskeletal Diseases, and Other Diseases. It is actively indicated for the treatment of Muscular Dystrophy, Duchenne, and Muscular Dystrophy, Facioscapulohumeral.