Scholar Rock Unveils Initial Lab Results Indicating Advantages of SRK-439 in Supporting Weight Reduction
Scholar Rock, a sophisticated biotech firm in its advanced phases that is concentrating on developing new therapeutic options for diseases such as spinal muscular atrophy, as well as conditions affecting the heart and metabolism, and other critical illnesses that are deeply influenced by protein growth factors, has publicized recent findings from preclinical studies. These findings suggest that SRK-439 may be capable of maintaining muscle mass and enhancing metabolic wellness in the context of weight reduction efforts that promote overall health.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The experimental findings indicate that SRK-439 enhances the retention of muscle mass as well as accelerates the reduction of adipose tissue when utilized in conjunction with a GLP-1 receptor agonist in mice with obesity caused by dietary factors. Furthermore, the application of SRK-439 contributed to a further decrease in fasting blood sugar levels in addition to the reduction observed with semaglutide on its own. Melissa Fulham, PhD, representing Scholar Rock, shared these in-depth outcomes at the Keystone Symposia dedicated to the study of Obesity: Causes and Consequences, held in Vancouver, BC, Canada, on the 5th of February.
“With evidence from preclinical studies that SRK-439 not only conserves muscle mass but also heightens the reduction of fat stores, we have a strong scientific foundation to explore the use of SRK-439 alongside GLP-1 RA treatments for the effective control of body weight,” remarked Jay Backstrom, M.D., MPH, the leading executive at Scholar Rock.
Dr. Backstrom added, “Our strategy to target myostatin with precise inhibition while pairing it with GLP-1 RA treatment holds promise in redefining approaches to weight loss management. We eagerly anticipate the commencement of our exploratory study involving apitegromab and GLP-1 RA therapy tailored for obesity. Concurrently, we are propelling the development of SRK-439 towards clinical evaluation.”
In the beginning of the year, Scholar Rock publicized the authorization from the U.S. Food and Drug Administration for their proposal to initiate a Phase 2 exploratory trial of apitegromab as an intervention for obesity in individuals who are concurrently using a GLP-1 RA. The study is anticipated to begin in the latter half of 2024, with expectations to gather trial data from the Phase 2 study of apitegromab by the middle of 2025. Scholar Rock is also progressing with the advancement of SRK-439, an innovative and selectively targeted myostatin inhibitor designed specifically for battling obesity.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
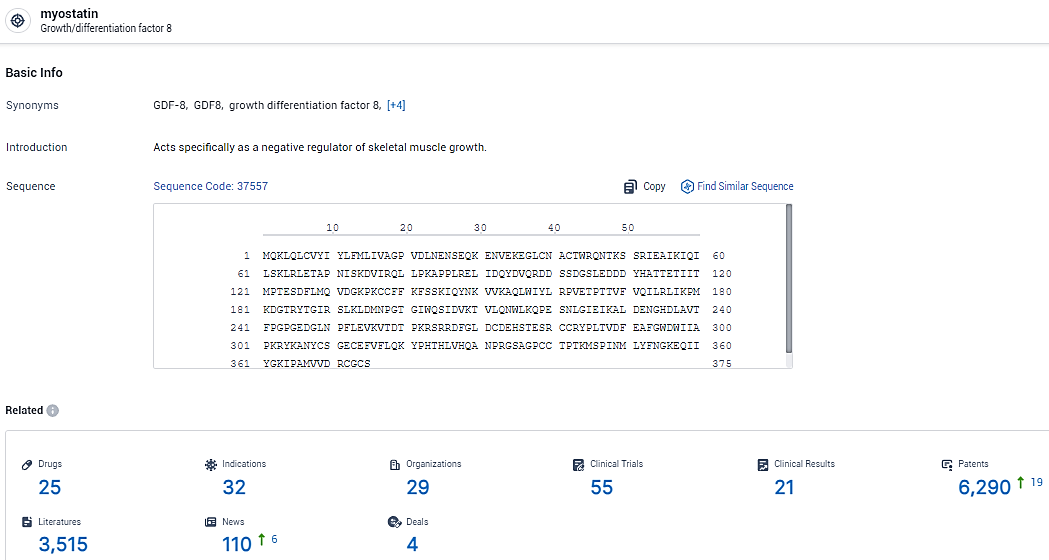 According to the data provided by the Synapse Database, As of February 18, 2024, there are 25 investigational drugs for the myostatin target, including 32 indications, 29 R&D institutions involved, with related clinical trials reaching 55, and as many as 6290 patents.
According to the data provided by the Synapse Database, As of February 18, 2024, there are 25 investigational drugs for the myostatin target, including 32 indications, 29 R&D institutions involved, with related clinical trials reaching 55, and as many as 6290 patents.
SRK-439 is a novel, preclinical, investigational myostatin inhibitor that has high in vitro affinity for pro- and latent myostatin and maintains myostatin specificity, and is initially being developed for the treatment of obesity. Based on preclinical data, SRK-439 has the potential to support healthier weight management by preserving lean mass. The efficacy and safety of SRK-439 have not been established and SRK-439 has not been approved for any use by the FDA or any other regulatory agency.