Positive Early Results of Rani Therapeutics' Phase 1 Trial for Oral RT-111
Rani Therapeutics Holdings, Inc., specializing in the oral administration of biological pharmaceuticals, recently reported encouraging preliminary outcomes from its initial phase clinical trial for RT-111. The RT-111, encapsulated in the proprietary RaniPill technology, carries a biologic similar to ustekinumab, known as CT-P43. During the trial, RT-111 showed a favorable safety profile, with participants absorbing the ustekinumab comparably effectively.
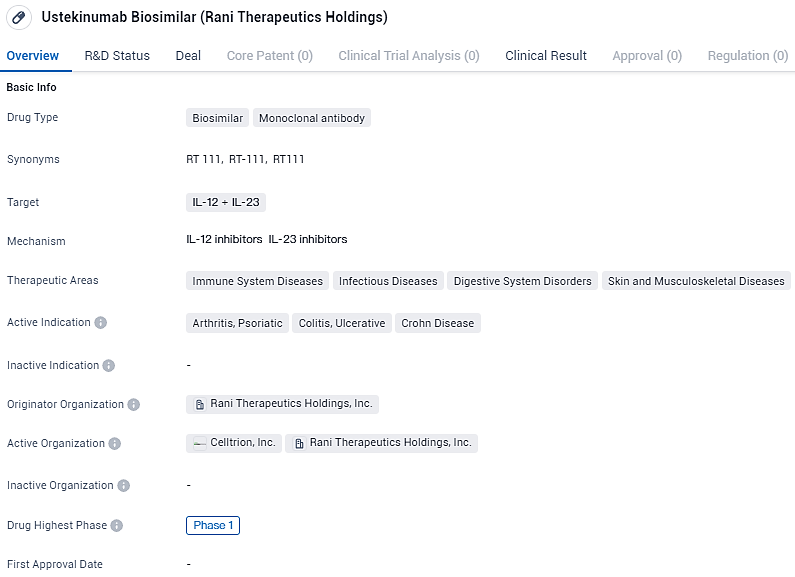
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Our team is inspired by the promising outcomes from the initial trial of RT-111, marking our third successful early-stage trial utilizing the innovative RaniPill technology. Talat Imran, the CEO of Rani Therapeutics, stated, “To our understanding, RT-111 represents the pioneering oral monoclonal antibody with a remarkable rate of bioavailability in human subjects.”
"We see RT-111 as a potential game-changer offering a unique treatment option for psoriasis patients," Imran explained. "This could be a pivotal alternative to the existing injectable biologics and orally consumed small molecule and peptide treatments. Achieving this Phase 1 trial's objectives is another testament to Rani's commitment to advancing oral biologic solutions for those managing autoimmune diseases."
Ustekinumab, a selective human IgG1қ monoclonal antibody, targets the p40 protein component shared by cytokines interleukin-12 and interleukin-23 (IL-12 and IL-23). As of now, patients can access ustekinumab solely through subcutaneous injections, and it is distributed under the brand name STELARA by Janssen in the U.S. for various conditions that include moderate to severe plaque psoriasis, active psoriatic arthritis, moderate to severe Crohn’s disease, and moderate to severe ulcerative colitis. Notably, there is a significant demand for an oral form of treatment for these conditions.
The exploratory Phase 1 clinical trial of RT-111 by Rani was hosted at a single center with an open-label framework in Australia. This early-phase study focused on assessing the safety, tolerability, and pharmacokinetics of RT-111 when administered to healthy adults. The trial included two separate groups for the RT-111, with doses of 0.5mg and 0.75mg, involving 20 participants each, and a comparative group of 15 individuals receiving a 0.5mg subcutaneous injection of STELARA.
Regarding sales, STELARA achieved an impressive revenue, with approximately $6.4 billion generated in the U.S. market and around $9.7 billion globally in the year 2022.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
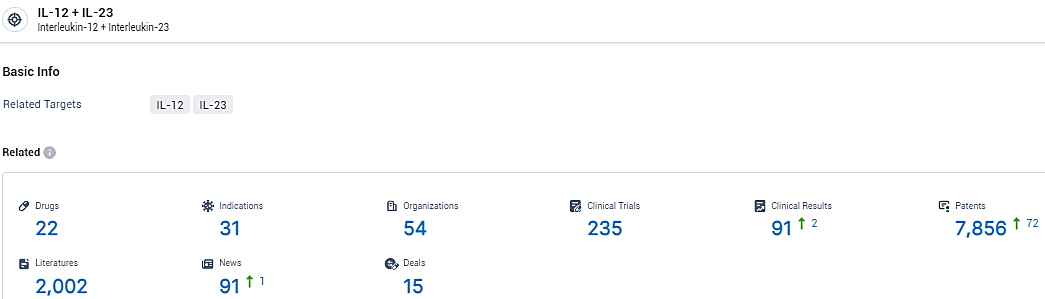
According to the data provided by the Synapse Database, As of February 18, 2024, there are 22 investigational drugs for the IL-12 and IL-23 target, including 31 indications, 54 R&D institutions involved, with related clinical trials reaching 235, and as many as 7856 patents.
RT-111 targets IL-12 and IL-23 and is intended for the treatment of various immune system diseases, infectious diseases, digestive system disorders, as well as skin and musculoskeletal diseases. The active indications for this drug include arthritis, psoriatic, colitis, ulcerative, and Crohn's disease. Currently, Ustekinumab Biosimilar has reached Phase 1 of clinical development, indicating progress in its evaluation and potential future availability for patients.