Sensei Biotherapeutics presents positive trial results for SNS-101 at SITC Conference 2023
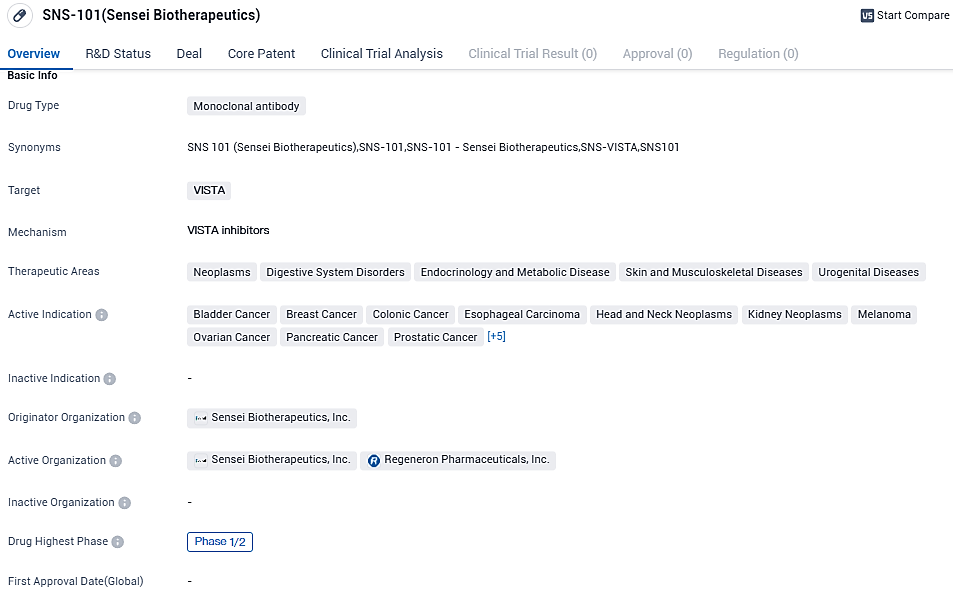
Clinical development immuno-oncology company, Sensei Biotherapeutics, Inc., which concentrates on discovering and creating the forthcoming generation treatments for cancer patients, unveiled early results from the lone therapy dose-increase segment of its Phase 1/2 clinical research for SNS-101, a selectively active, human monoclonal antibody directed at the immune checkpoint VISTA.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The information, set to be revealed during a late-breaker poster session at the Society for Immunotherapy of Cancer 38th Annual Meeting, suggests an optimal safety and pharmacokinetic profile among VISTA-blocking antibodies. It also indicates the capability to tackle long-term pharmacological problems that the first generation of VISTA-blocking methodologies have faced.
John Celebi, CEO and President of Sensei Biotherapeutics, expressed his gratification over the promising clinical data for SNS-101, a revolutionary VISTA-blocking antibody which proves the effectiveness of their conditionally active methodology.
The details affirm e that this innovative antibody handled all current dosage levels without complications, shows dose-dependent linear pharmacokinetics expected preclinically to cause immune system-controlled anti-tumor activity, along with a cytokine profile consistent with no occurrence of cytokine release syndrome.
John Celebi further explained that the clinical study data so far provides significant initial proof that SNS-101 may provide clinically significant and mechanistic distinction from first generation anti-VISTA methods. This is signified by SNS-101 dosage levels which are at least 10 times higher than the first clinical study of a competing VISTA antibody that was abruptly stopped due to cytokine release syndrome and unfavorable pharmacokinetics.
The multi-center Phase 1/2 clinical trial is being conducted to assess SNS-101’s safety, tolerance, pharmacokinetics, pharmacodynamics, and efficacy as a single and in conjunction with Regeneron’s PD-1 inhibitor Libtayo® (cemiplimab) in patients with advanced solid tumors.
Medical oncologist at NEXT Oncology, Shiraj Sen, M.D., Ph.D., said he was optimistic about the SNS-101 trial's patient experience so far, as it shows a potential leading safety profile and a tri-weekly dosage plan, which eases the logistical strain that agents that require more frequent administration usually place on patients, due to their unfavorable pharmacokinetics.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
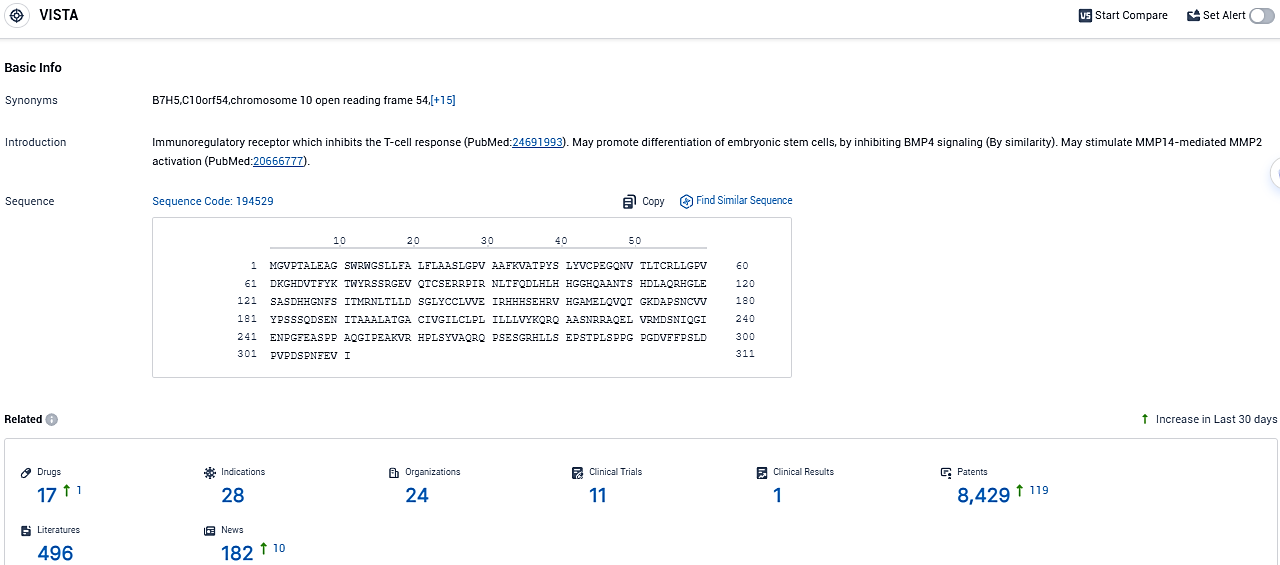
According to the data provided by the Synapse Database, As of November 12, 2023, there are 17 investigational drugs for the VISTA target, including 28 indications, 24 R&D institutions involved, with related clinical trials reaching 11 and as many as 8429 patents.
With its wide range of active indications, SNS-101 has the potential to address multiple therapeutic areas. However, as it is currently in Phase 1/2 of clinical development, further research and testing are needed to fully understand its safety and efficacy profile.