BDC-1001: Brief Review of its R&D progress and the clinical outcome in 2023 ESMO
On October 20, 2023, the first-in-human trial of BDC-1001 in advanced HER2-positive solid tumors was reported at the ESMO Congress.
BDC-1001's R&D Progress
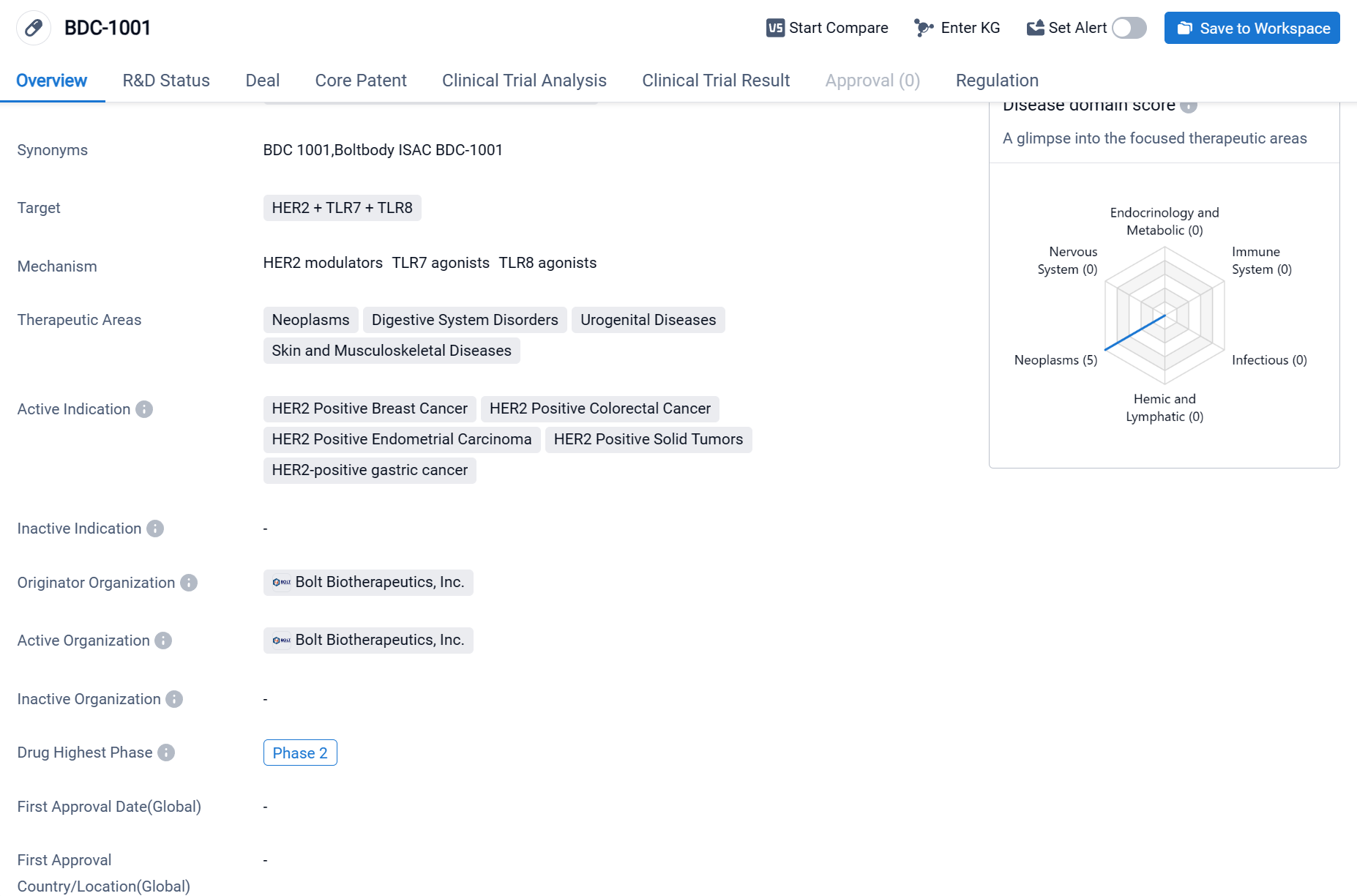
BDC-1001is an immune stimulating antibody conjugate (ISAC) drug developed by Bolt Biotherapeutics, Inc. The drug targets HER2, TLR7, and TLR8, making it potentially effective in treating various diseases. The therapeutic areas that BDC-1001 focuses on include neoplasms (abnormal growth of cells), digestive system disorders, urogenital diseases, and skin and musculoskeletal diseases.
According to the Patsnap Synapse, BDC-1001 is currently in Phase 2, which is the highest phase of clinical trials globally. And the clinical trial areas for BDC-1001 are primarily in the United States, South Korea, and Spain. The key indication is HER2 Positive Breast Cancer.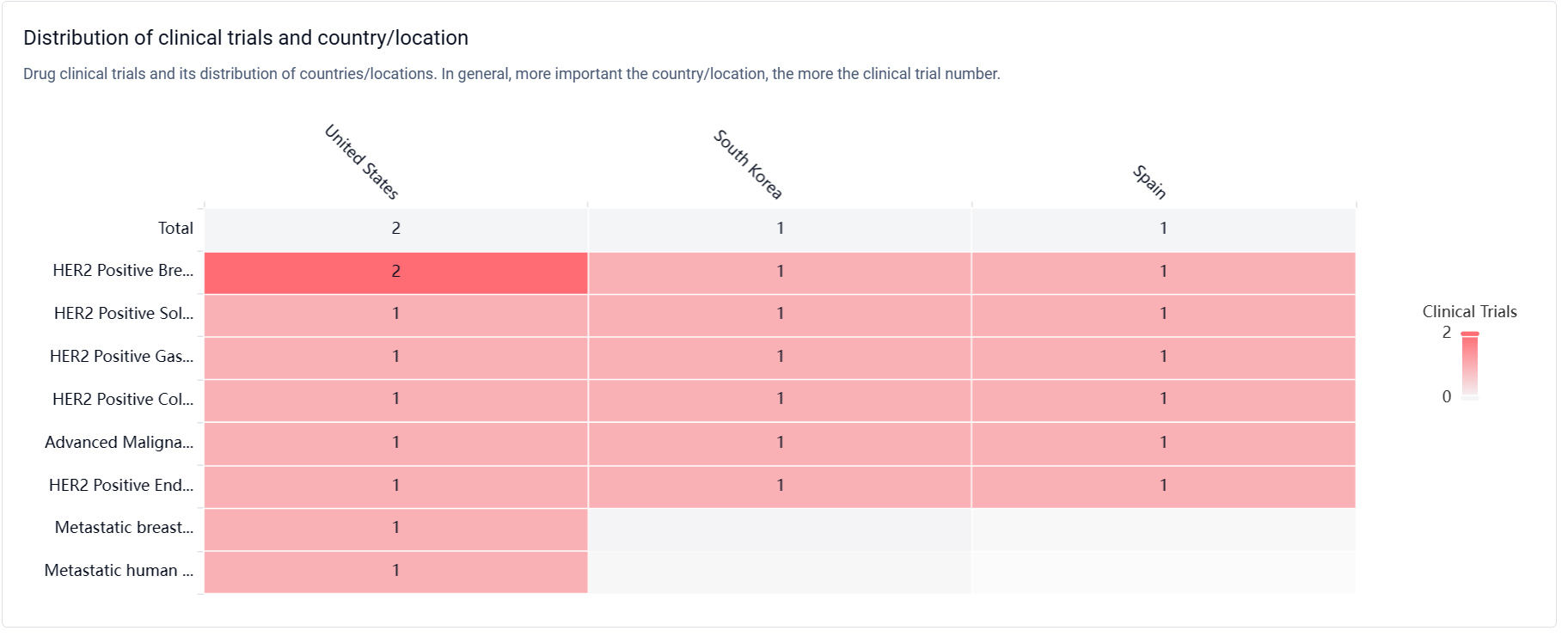
Detailed Clinical Outcome of BDC-1001
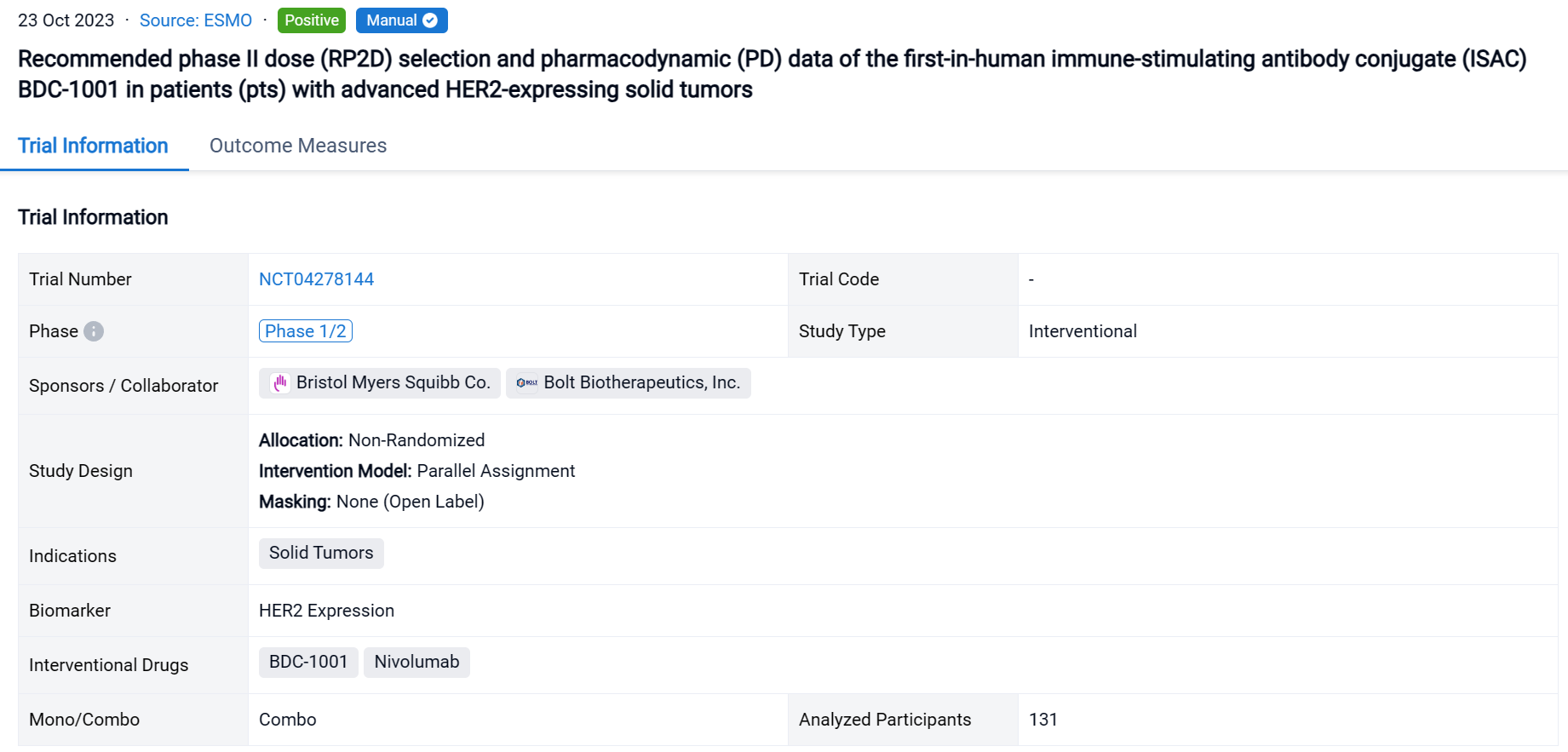
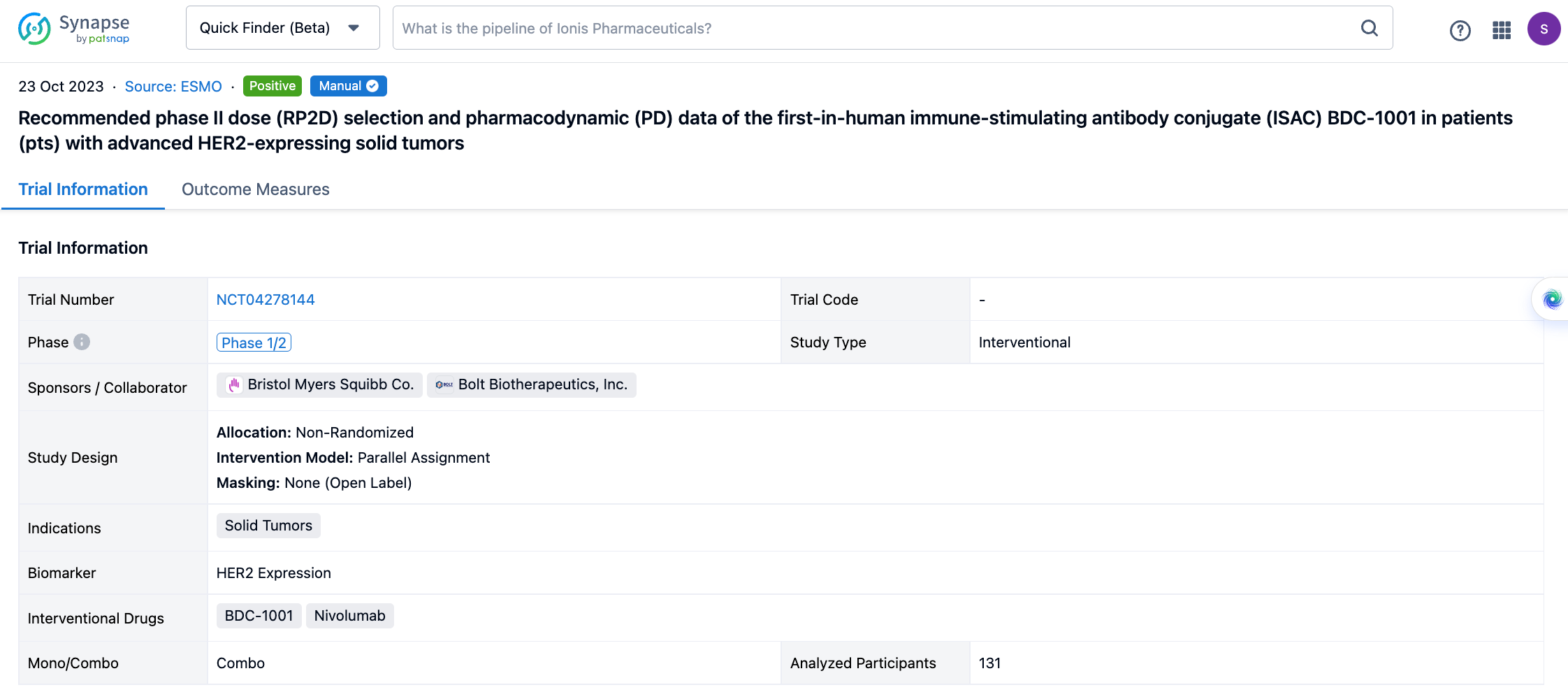
The non-randomized, parallel assignment, open-labeled clinical trial(NCT04278144) was aimed to recommend phaseⅡdose selection and pharmacodynamic data of the immune-stimulating antibody conjugate (ISAC) BDC-1001 in patients with advanced HER2-expressing solid tumors.
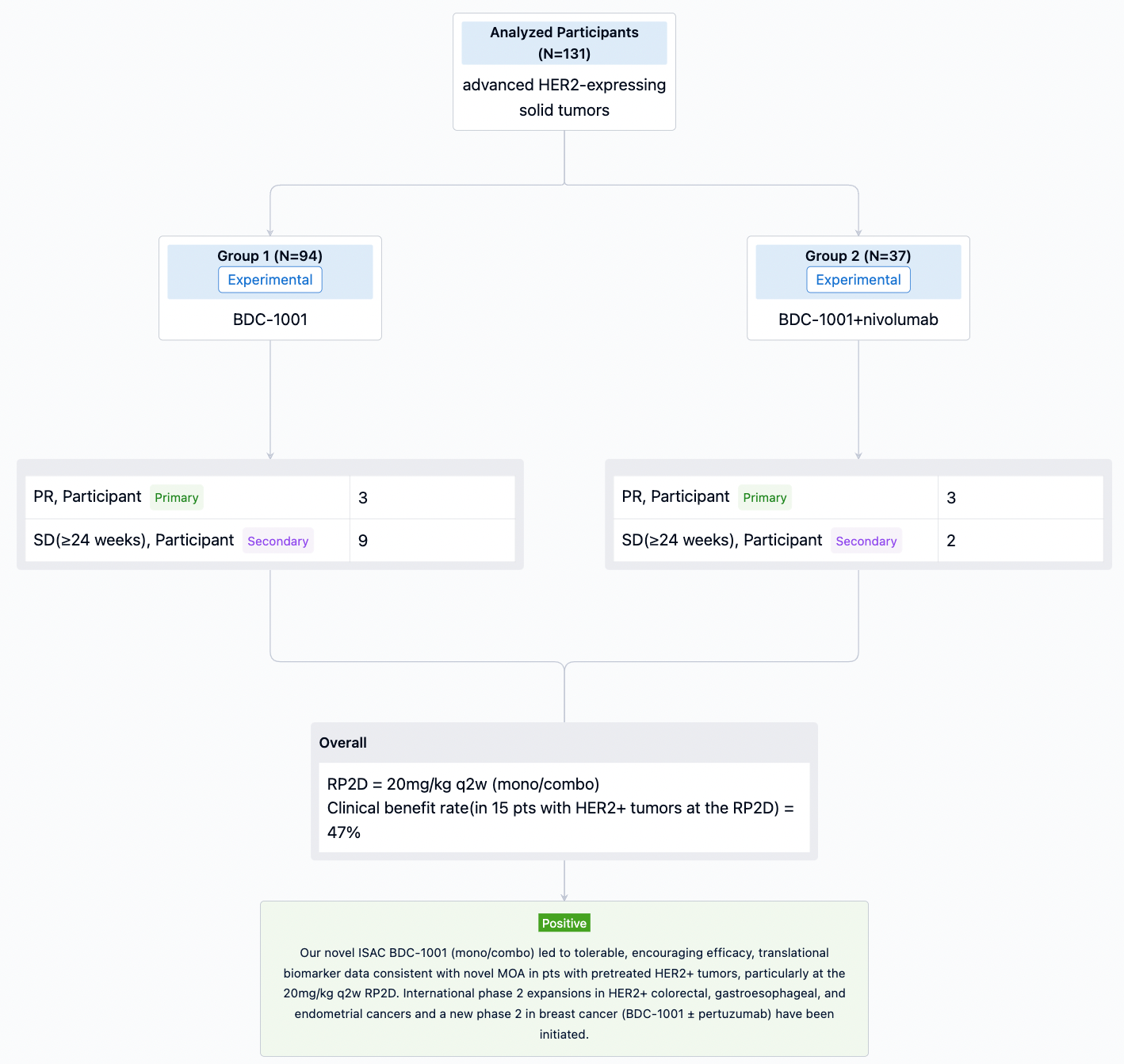
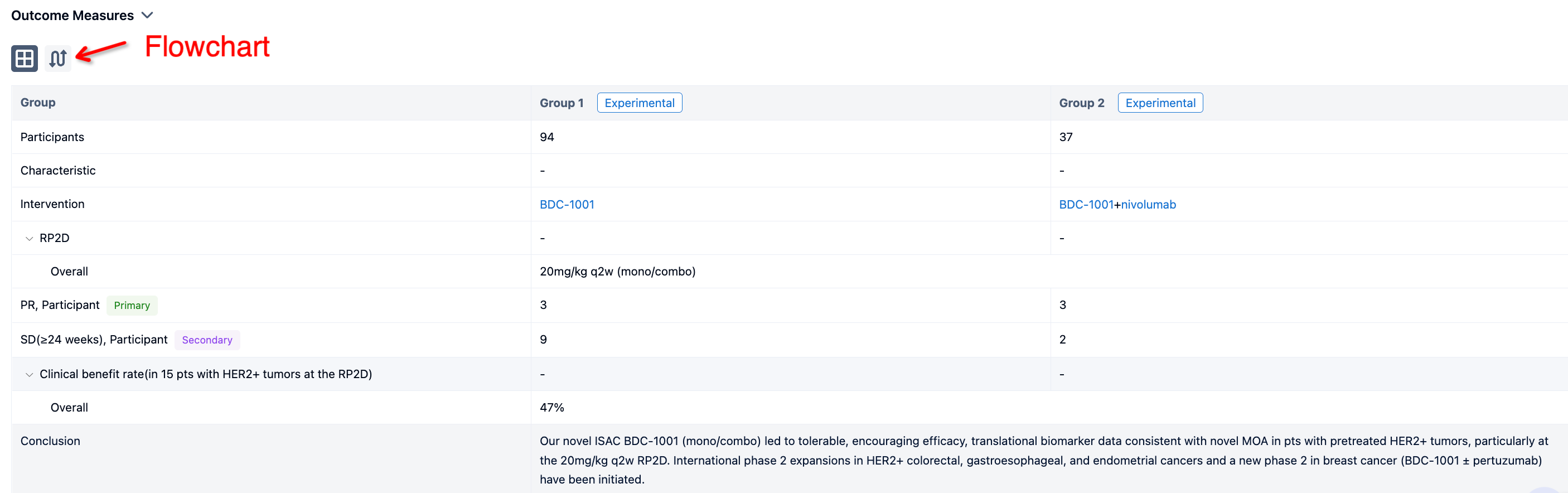
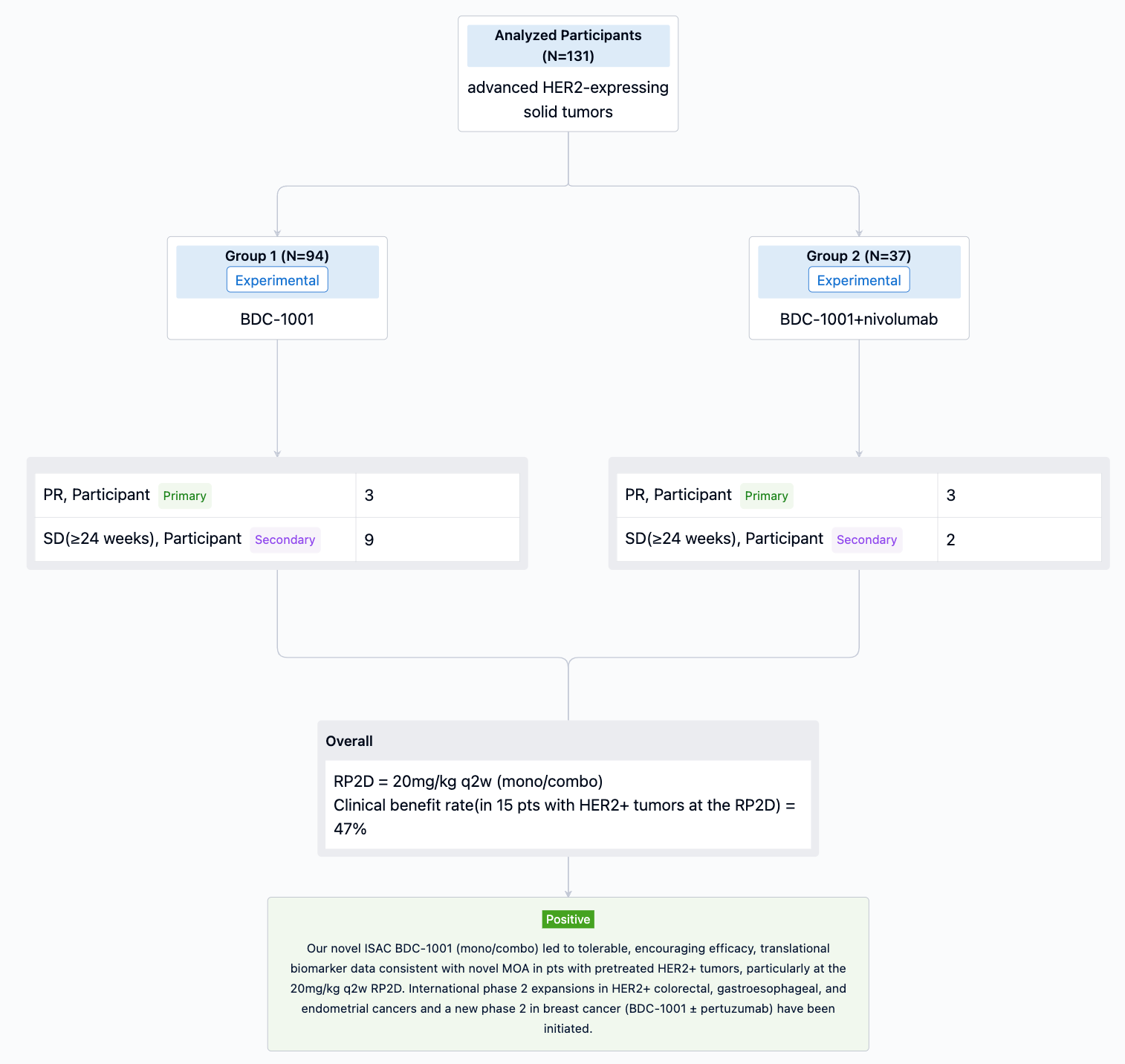
In this study, Pts with HER2+ (protein or gene) or HER2-low solid tumors progressing after standard therapies (Txs) were enrolled. BDC-1001 was given IV q3w, q2w, or q1w as monotherapy (mono; n=94) and q2w or q1w with nivolumab 240mg q2w (combo; n=37).
The result showed that As of 24 March 2023, 131 pts with 16 different tumor types received 0.15 to 20mg/kg of BDC-1001. Mean age 60 yrs, median 4 prior lines of Txs (range 0-13; prior anti-HER2 67%, immune 29%). The RP2D of 20mg/kg q2w (mono/combo) was determined based on safety, efficacy, pharmacokinetic, and PD data. BDC-1001 was well tolerated as mono/combo. Grade 1/2 infusion-related reactions were the most common adverse events (30%). Clinical activity observed in multiple tumor types and doses improved as targeted Cmin ≥10mg/mL was reached, particularly at the RP2D. 6 pts (3 mono) had partial response (PR), 11 pts (9 mono) achieved stable disease (SD) ≥24 wks. Clinical benefit rate in 15 pts with HER2+ tumors at the RP2D was 47% (27% confirmed PR, additional 20% SD ≥24wks), 60% tumor shrinkage and 33% pts still on active Tx. Comprehensive plasma and fresh biopsies (bx) studies performed. PD responses in plasma and paired tumor bx (protein/gene analyses) demonstrated immune activation with BDC-1001 (e.g., IFNg, antigen processing, macrophage activation), not observed by trastuzumab treatment, and subsequent T cell recruitment in tumor tissue.
It can be concluded that our novel ISAC BDC-1001 (mono/combo) led to tolerable, encouraging efficacy, translational biomarker data consistent with novel MOA in pts with pretreated HER2+ tumors, particularly at the 20mg/kg q2w RP2D. International phase 2 expansions in HER2+ colorectal, gastroesophageal, and endometrial cancers and a new phase 2 in breast cancer (BDC-1001 ± pertuzumab) have been initiated.
How to Easily View the Clinical Results Using Synapse Database?
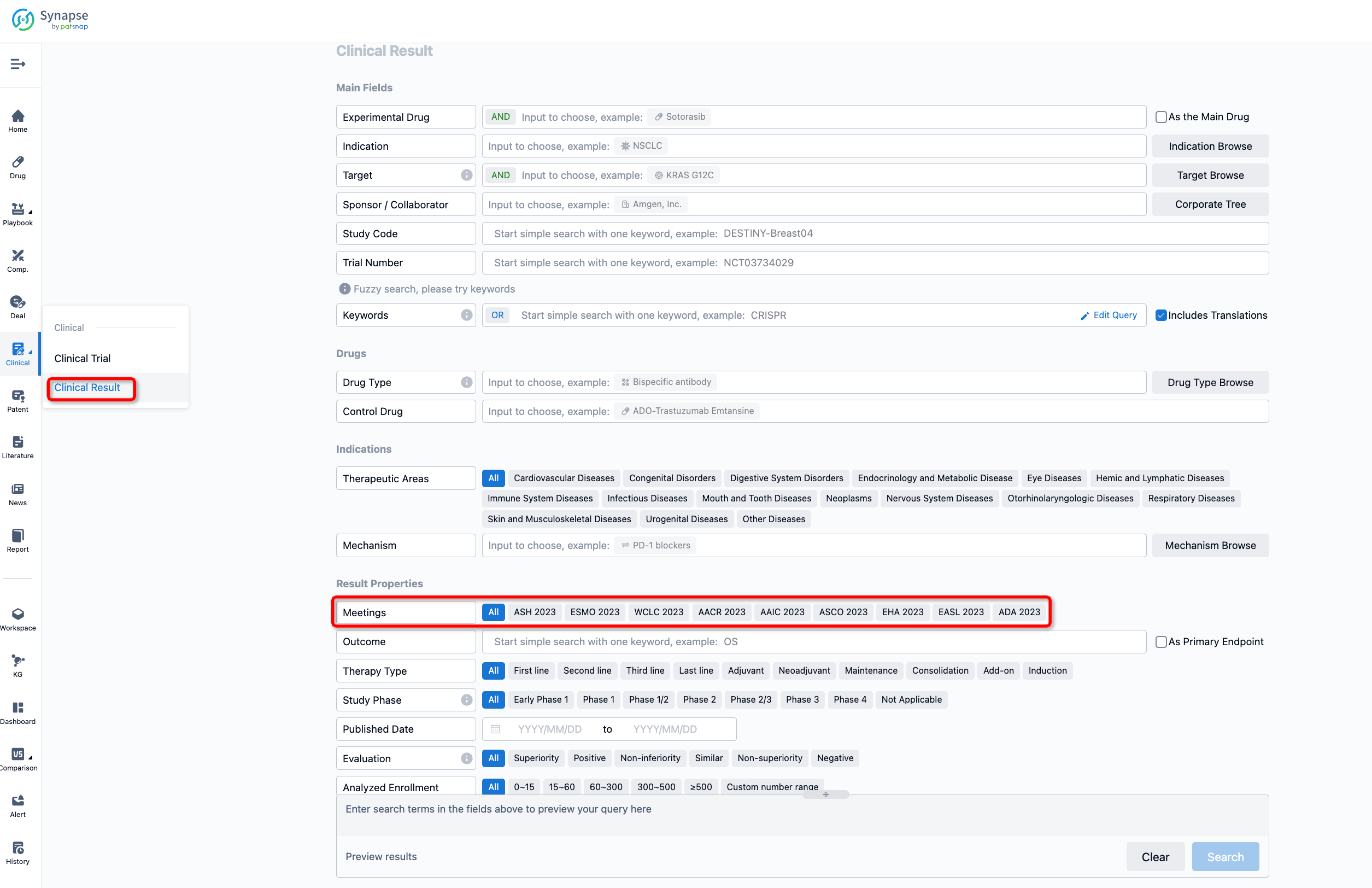
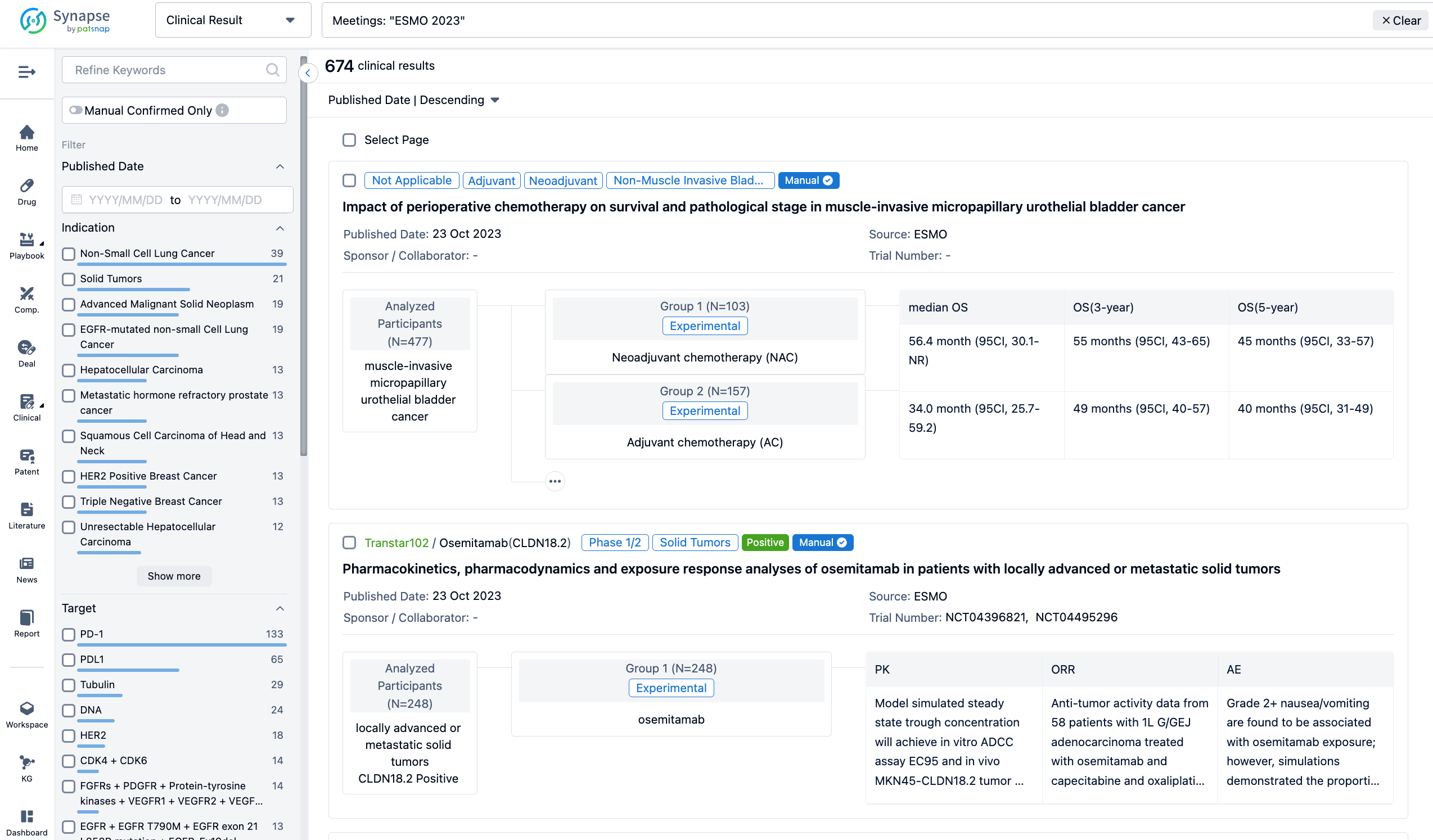
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
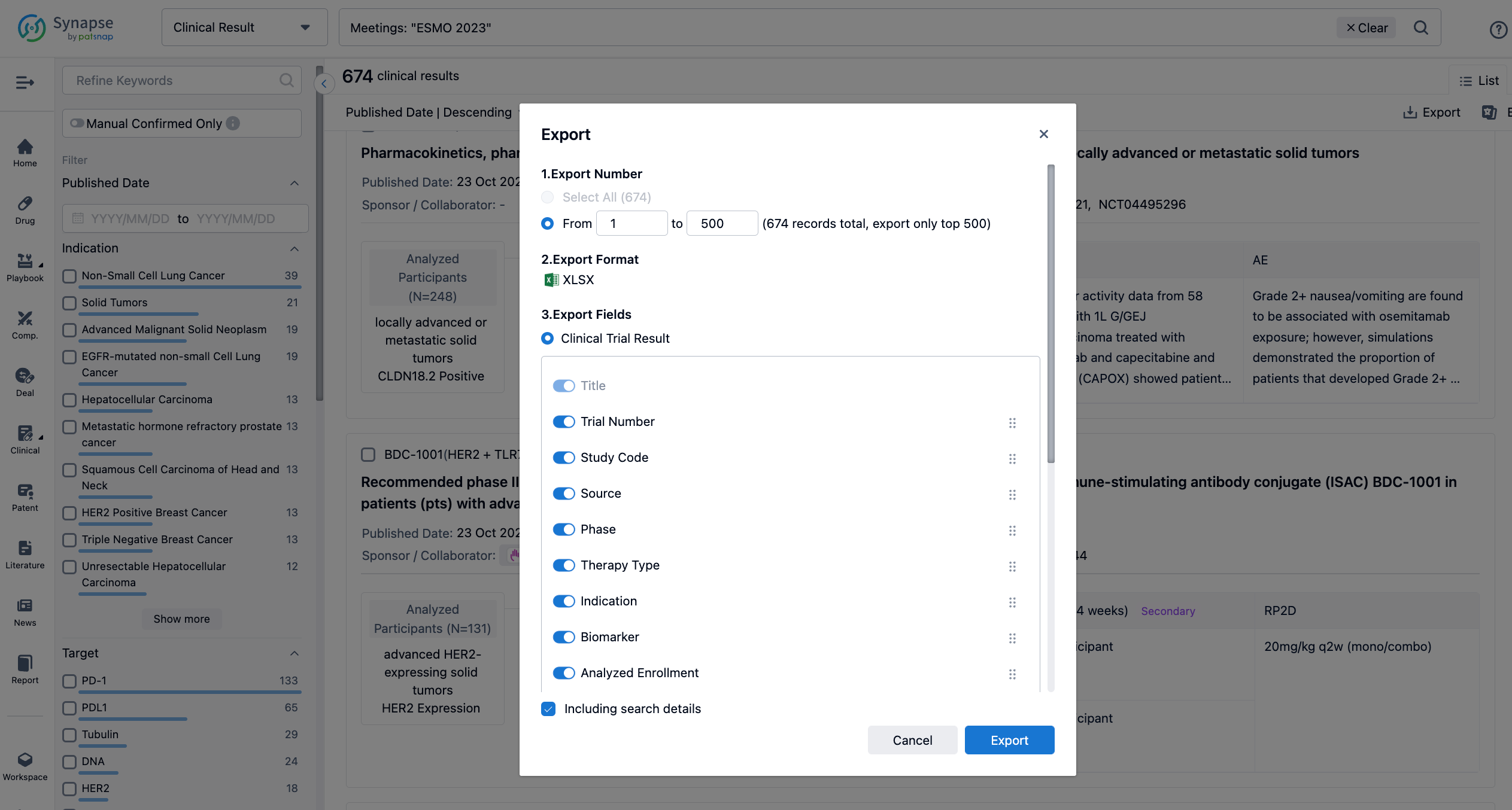
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!