Shanghai Junshi Biosciences' anti-PD-1 monoclonal antibody, Toripalimab, announces Phase 3 trial data for the treatment of small-cell lung cancer
At the 2023 European Society for Medical Oncology (ESMO) congress, a phase III randomized, double-blind, placebo-controlled, multi-center clinical trial, EXTENTORCH, of Shanghai Junshi Biosciences' anti-PD-1 monoclonal antibody toripalimab, was selected for the Late-breaking Abstract (LBA). This trial aims to compare the efficacy and safety of toripalimab or placebo combined with first-line treatment Etoposide and platinum for extensive-stage small-cell lung cancer (ES-SCLC).
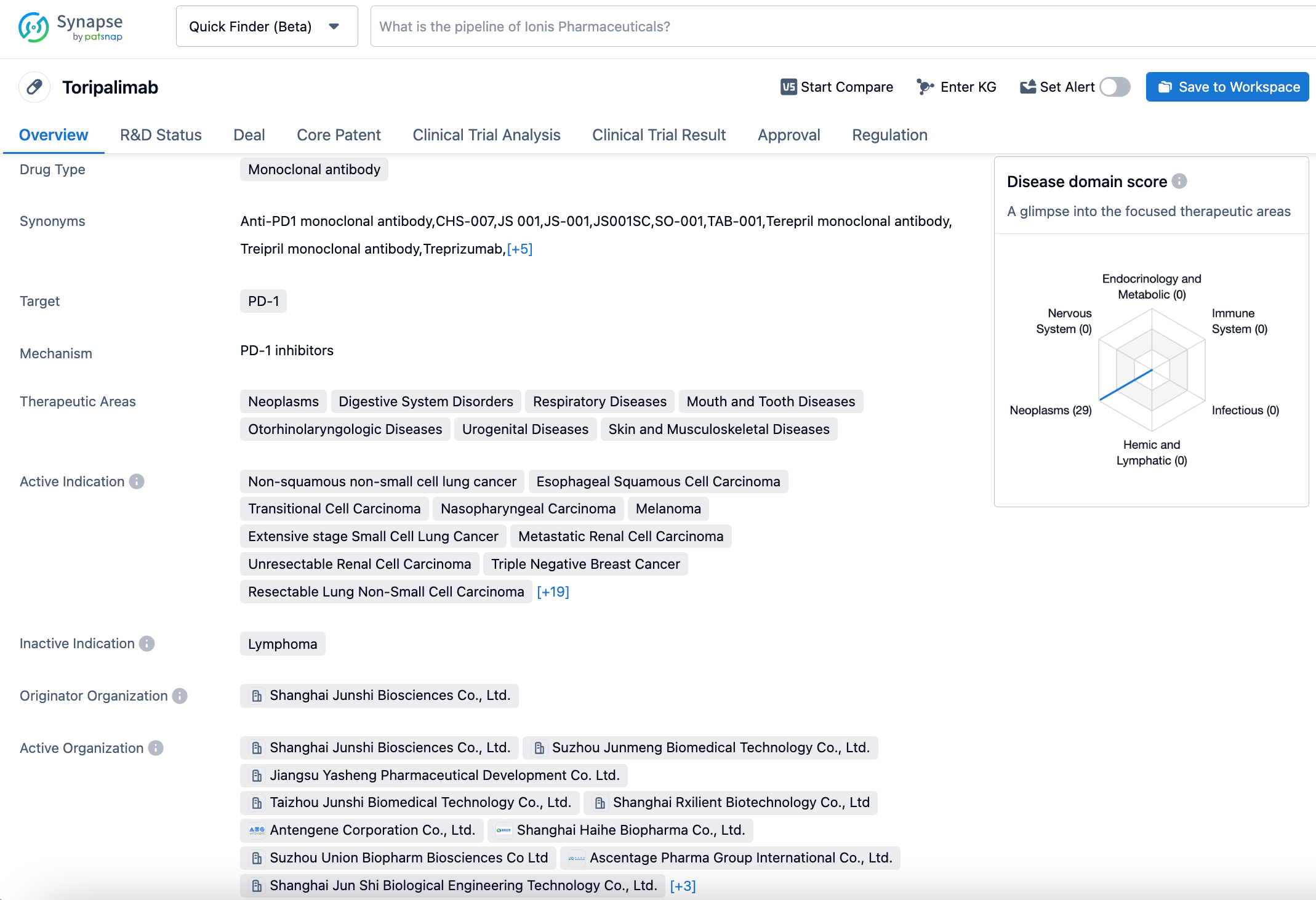
Toripalimab, independently developed by Junshi Biosciences, is used for the treatment of various malignant tumors. To date, over 40 clinical trials initiated by Junshi Biosciences covering more than 15 indications have been carried out globally, including in China, the U.S., Southeast Asia, and Europe. So far, the drug has been approved in China for six indications, including melanoma, nasopharyngeal carcinoma, urothelial cancer, esophageal squamous cell carcinoma, and non-squamous non-small cell lung cancer. In terms of internationalization, toripalimab has received two breakthrough therapy designations, one fast track designation, one priority review designation, and five orphan drug designations from the U.S. Food and Drug Administration (FDA) in the fields of mucosal melanoma, nasopharyngeal carcinoma, soft tissue sarcoma, esophageal cancer, and small cell lung cancer.
The MELATORCH study (NCT03430297) is a randomized, controlled, multi-center phase III clinical trial that aimed to compare the efficacy and safety of toripalimab versus Dacarbazine in patients with unresectable or metastatic melanoma who had not previously received systemic anticancer treatment. The results showed that toripalimab significantly prolonged the PFS of patients with unresectable or metastatic melanoma as first-line treatment compared to Dacarbazine. The safety data of toripalimab were similar to previous studies with no new safety signals found. Detailed data from this study will be presented at subsequent international academic conferences.
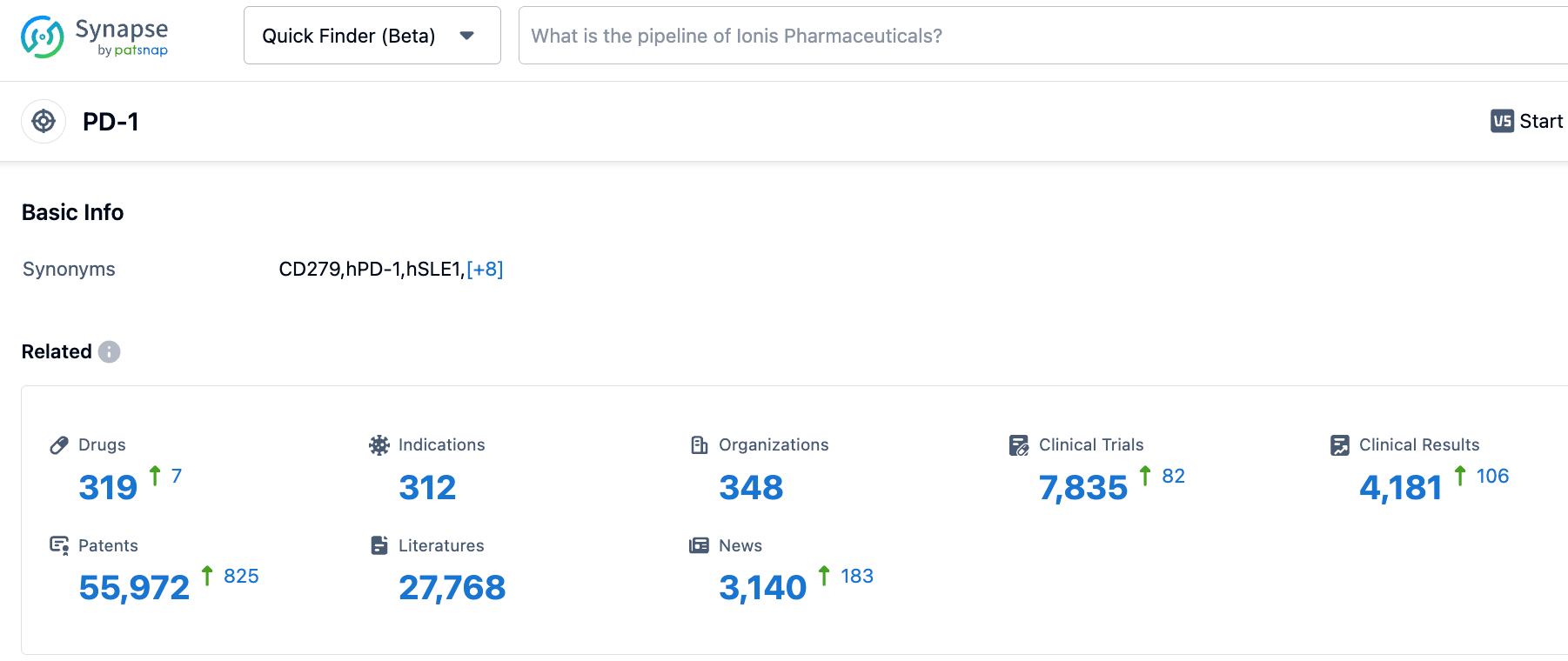
According to information disclosed by the Synapse database, as of October 21, 2023, there are 318 investigational drugs targeting PD-1, covering 312 indications, from 346 institutions, involving 7,826 related clinical trials, and up to 55,993 patents.