Encouraging Phase I Results for HUADONG MEDICINE'S Oral GLP-1 Receptor Agonist HDM1002
HUADONG MEDICINE has provided an update through its fully-owned subsidiary, Hangzhou Zhongmei HuaDong Pharmaceutical CO. LTD, revealing encouraging findings from Phase I clinical trials of its new oral small molecule GLP-1 receptor agonist, HDM1002, conducted in China.
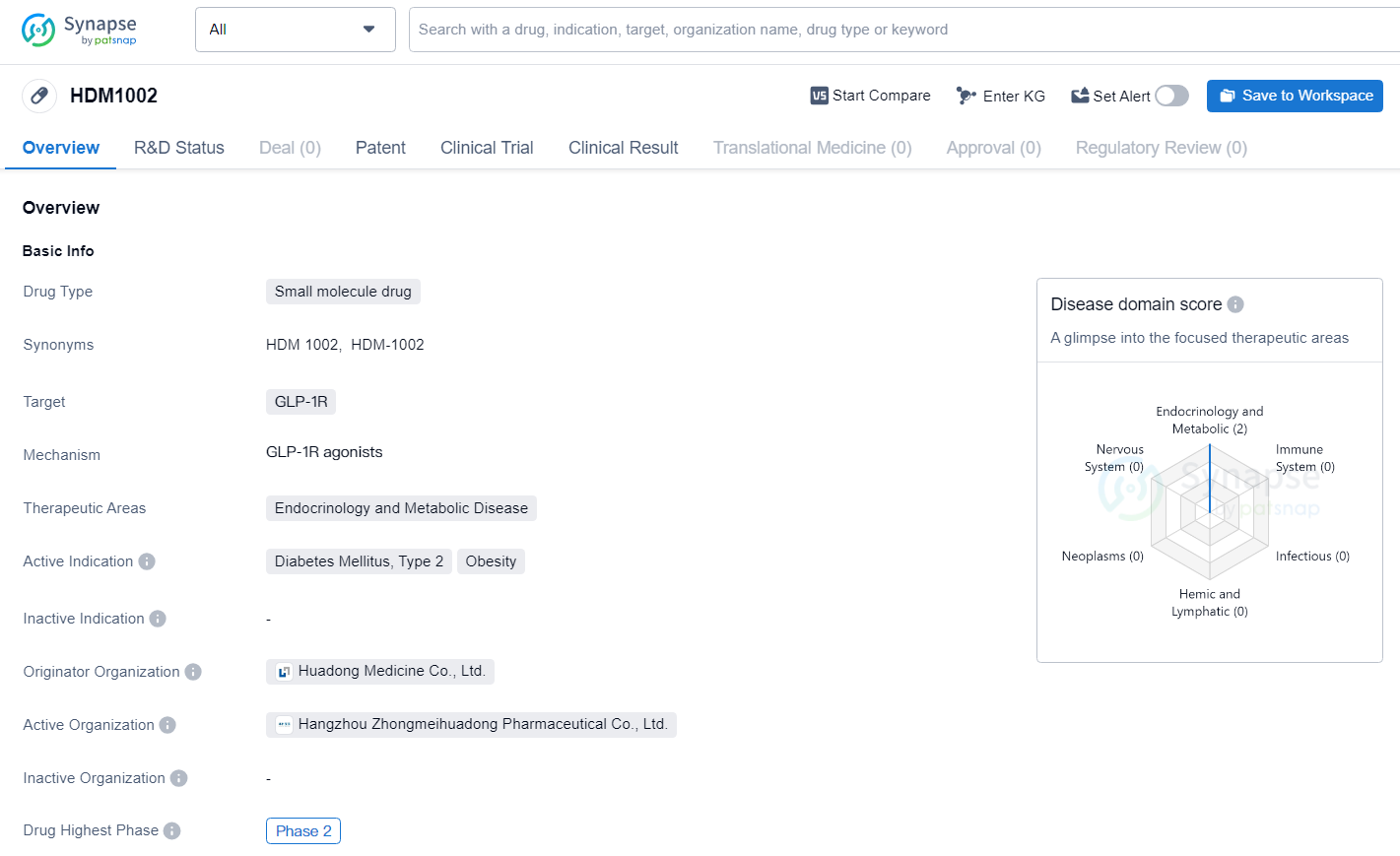
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The Phase Ia clinical trial of HDM1002 conducted in China employed a randomized, double-blind, placebo-controlled design to assess the safety, tolerability, and pharmacokinetics of single, escalating oral doses of HDM1002 in healthy adults. A total of 79 healthy participants were included in the study. The findings revealed that HDM1002 displayed linear pharmacokinetics across a dose range of 10mg to 600mg, with the drug showing good safety and tolerability profiles. All observed adverse events were rated as grade 1 or 2. Notably, a single dose of HDM1002 significantly lowered postprandial blood glucose levels in healthy subjects compared to the placebo, with a clear dose-dependent trend. Furthermore, high-fat meals did not affect the pharmacokinetic properties of HDM1002.
The Phase Ib clinical trial of HDM1002 in China followed a similar randomized, double-blind, placebo-controlled design to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of multiple, escalating oral doses of HDM1002 in overweight and obese adults. Sixty participants took part in this trial. After 28 days of continuous treatment with doses ranging from 50mg to 400mg, HDM1002 showed good safety and tolerability. The most frequently reported adverse events were gastrointestinal in nature, predominantly mild nausea and vomiting. Subjects receiving 100mg or higher doses demonstrated significantly greater weight loss compared to the placebo group on day 28, also showing a dose-dependent effect. Participants within the target dose range experienced an average weight reduction of 4.9% to 6.8% from baseline by day 28.
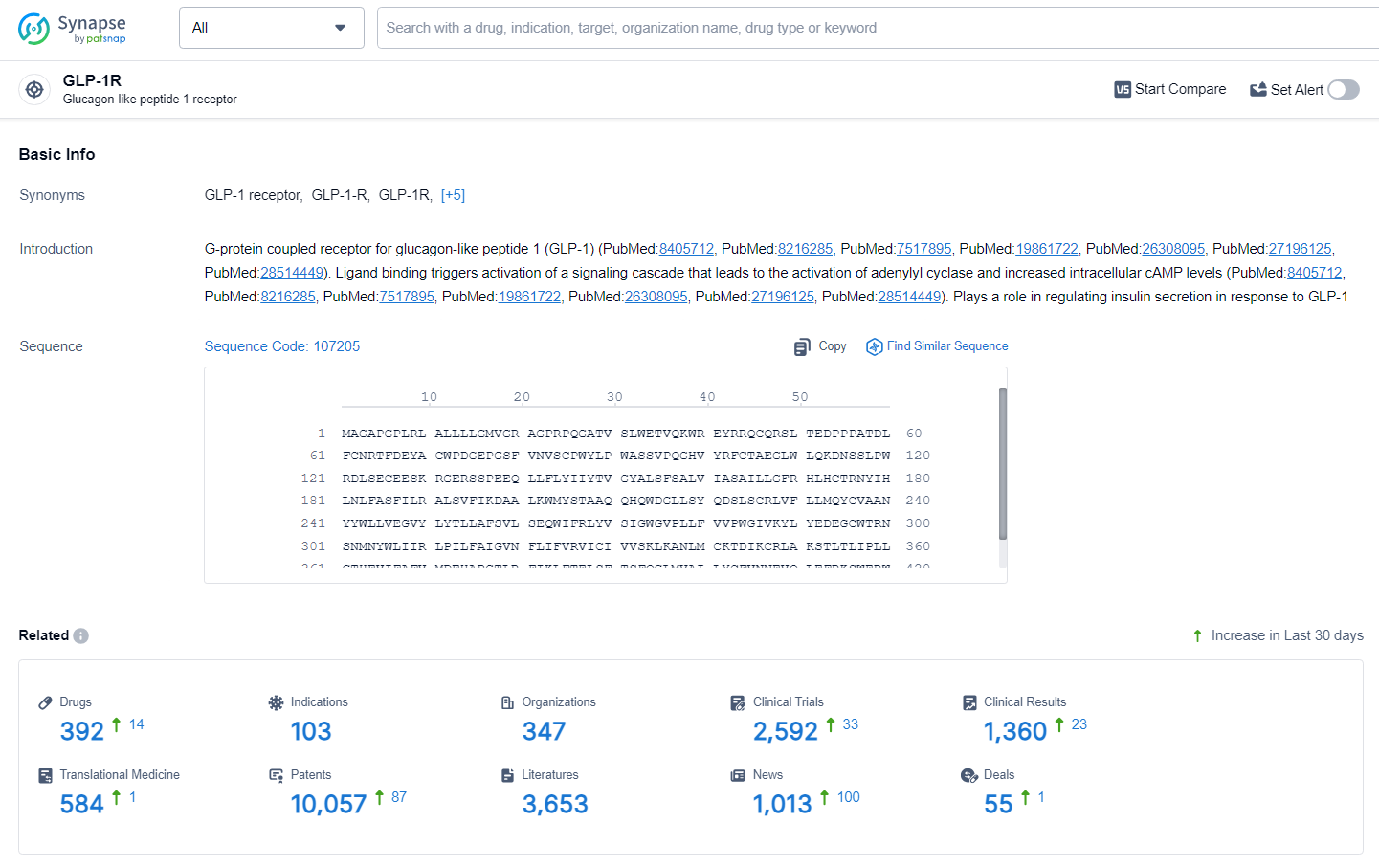
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of July 1, 2024, there are 392 investigational drugs for the GLP-1R target, including 103 indications, 347 R&D institutions involved, with related clinical trials reaching 2592, and as many as 10057 patents.
HDM1002 targets GLP-1R for the treatment of Diabetes Mellitus, Type 2, and Obesity. The drug has reached Phase 2 in both global and Chinese trials, indicating promising early-stage results and potential for addressing significant unmet medical needs in the field of endocrinology.