Sionna Therapeutics Reveals Start of First Stage Clinical Trials with SION-109 for Treating Cystic Fibrosis
Sionna Therapeutics, a biopharmaceutical firm focused on creating unique and potent therapies for cystic fibrosis, has revealed the administration of the initial dose to a healthy participant in the Stage 1 clinical study for SION-109. This step follows the U.S. Food and Drug Administration's approval of its Investigational New Drug submission.
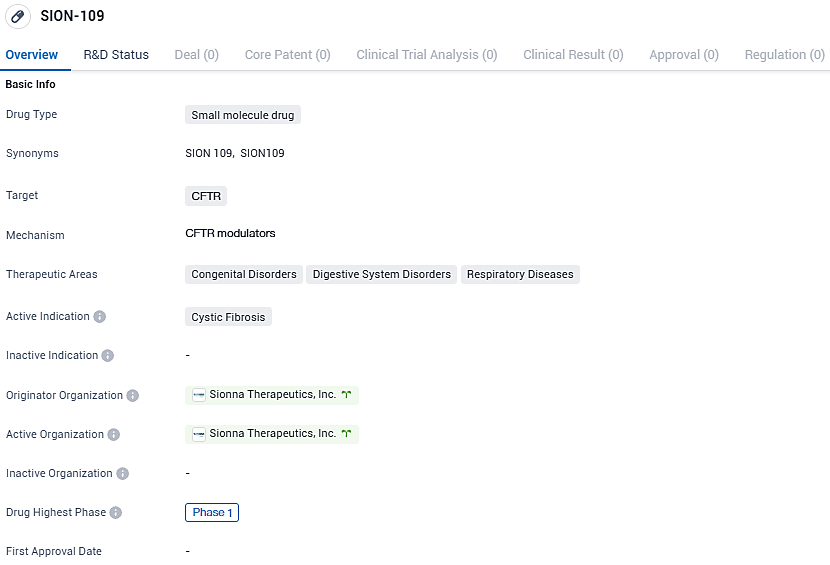
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
SION-109 is a novel small molecule crafted to interact with the binding site formed by the interplay of intracellular loop 4 and the premier nucleotide-binding domain of the CFTR protein. The strategy behind SION-109 is to augment the range of compounds already in development by the company, which focus on directly engaging NBD1, amongst which includes SION-638, a leading candidate for NBD1 stabilization, currently undergoing Phase 1 trials.
"The venture into correcting NBD1 is groundbreaking, and with the initiation of SION-109's clinical trials, we are at the brink of pioneering exclusive combination therapies that could ultimately result in comprehensive CFTR restoration," expressed Mike Cloonan, who helms Sionna as President and CEO.
Cloonan continued, "The progression of our secondary drug candidate to Phase 1 clinical trials represents a milestone that our R&D personnel have reached through unrelenting dedication. Their extensive expertise in CF, coupled with an earnest commitment to rapidly advance these programs, is geared towards introducing novel treatment alternatives for those affected by CF and their loved ones."
CF arises from genetic aberrations in the CFTR protein, which acts as an epithelial ion channel crucial for regulating the consistency of mucus across the respiratory system, gastrointestinal tract, and other vital organs. The predominant CFTR mutation, known as ΔF508, leads to a destabilized NBD1 at physiological temperature, critically undermining the CFTR's functional integrity. Targeting ancillary sites such as ICL4 could pave the way for combinative therapeutic approaches aimed at comprehensive CFTR remediation. Statistics suggest that the ΔF508 mutation is present in about 90 percent of the CF population.
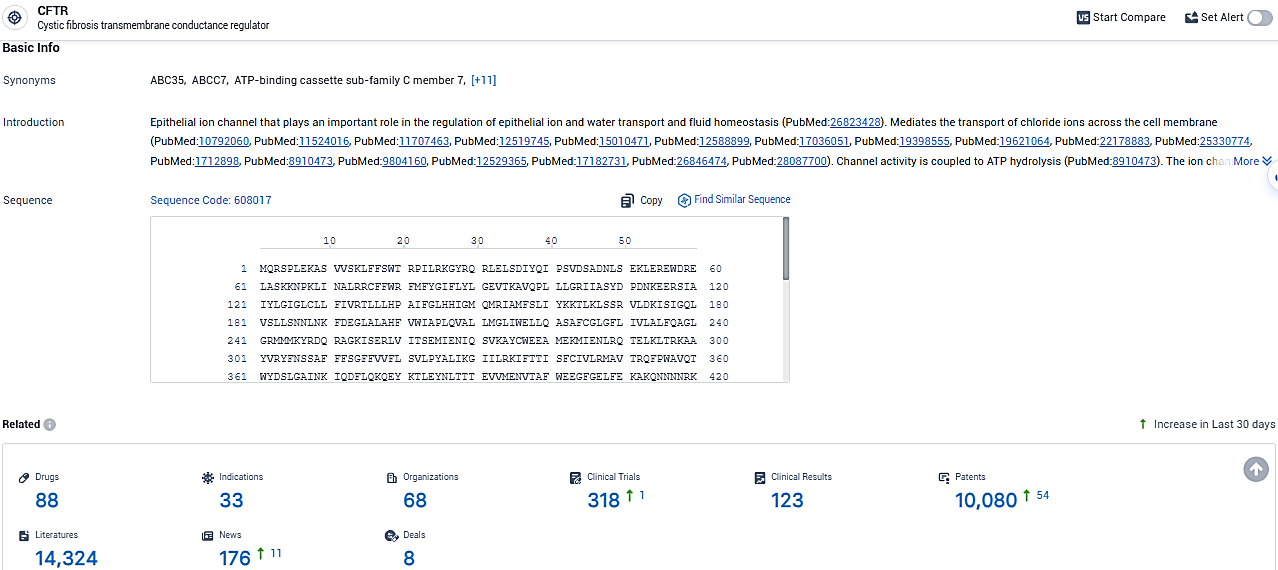
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of January 30, 2024, there are 88 investigational drugs for the CFTR target, including 33 indications, 68 R&D institutions involved, with related clinical trials reaching 318, and as many as 10080 patents.
SION-109 targets the CFTR protein and has the potential to address the symptoms and complications associated with this genetic disorder. While still in the early stages of development, SION-109 shows promise in the field of biomedicine and may have applications in other therapeutic areas as well.