Sciwind Biosciences Announces Promising Initial Clinical Results for Oral, Sustained-Release GLP-1 Mimetic Ecnoglutide, XW004
Sciwind Biosciences Co., Ltd., a biopharmaceutical enterprise in the clinical phase dedicated to advancing novel treatments for metabolic disorders, has reported encouraging preliminary findings from the initial four groups in a Phase 1 clinical study for the orally administered ecnoglutide (XW004). Ecnoglutide stands out as a durable, preferentially activating cAMP pathway, glucagon-like peptide-1 (GLP-1) mimic, under development for managing type 2 diabetes and excessive weight. Formulated as an oral tablet, XW004 delivers ecnoglutide for patient use.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The initial phase study was a structured, blinded trial involving randomization, managing a multiple escalating dose framework, and included a placebo comparator. This study admitted 42 individuals in good health condition and an additional 14 participants with obesity, all being from Australia. The recruited individuals were assigned in a random manner to either receive a placebo or the test compound XW004, in the form of a daily oral pill.
For the first three groups, known as Cohorts 1 to 3, the designated dosage amounts of XW004 were set at 7 mg, 15 mg, and 30 mg daily for a duration of two weeks. For the fourth group, Cohort 4, the aim was to administer 30 mg of XW004 daily across a span of six weeks. The treatment phases were designed to include gradual increments leading up to the target dosage. Over the course of the trial, the primary aspects assessed were safety profiles, how well participants could tolerate the treatment, the drug's pharmacokinetic attributes, and the average alteration in body weight compared to initial measurements.
The overall assessment of the treatment's safety and how well it was tolerated when administered orally, which is known as oral ecnoglutide, was found to align with what is expected of GLP-1 peptide agonist compounds. Adverse reactions that were commonly reported consisted of symptoms like nausea, headaches, bouts of diarrhea, instances of vomiting, and a reduced desire to eat. The severity of these adverse events ranged from mild to moderate and occurred predominantly while dosages were being scaled up.
"The initial results showcasing XW004's safety, potency, and pharmacokinetic responses are highly promising. Notably, the marked reduction in body weight following brief treatment suggests a strong case for oral ecnoglutide's further development as an option for treating obesity and type 2 diabetes," commented Hai Pan, the establishing mind and Chief Executive Officer at Sciwind Biosciences.
Added Mohammed Junaidi, MD, and the Vice President of Clinical Development at Sciwind, "We are optimistic that adopting dosing schedules that are less frequent could markedly encourage better adherence by patients, address production complexities, and enhance the availability of this category of medication to a diverse patient demographic. Should XW004 proceed to successful development, there is a possibility it could emerge as the inaugural GLP-1 receptor activator administered orally on a weekly basis, specifically aimed at metabolic disorders."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
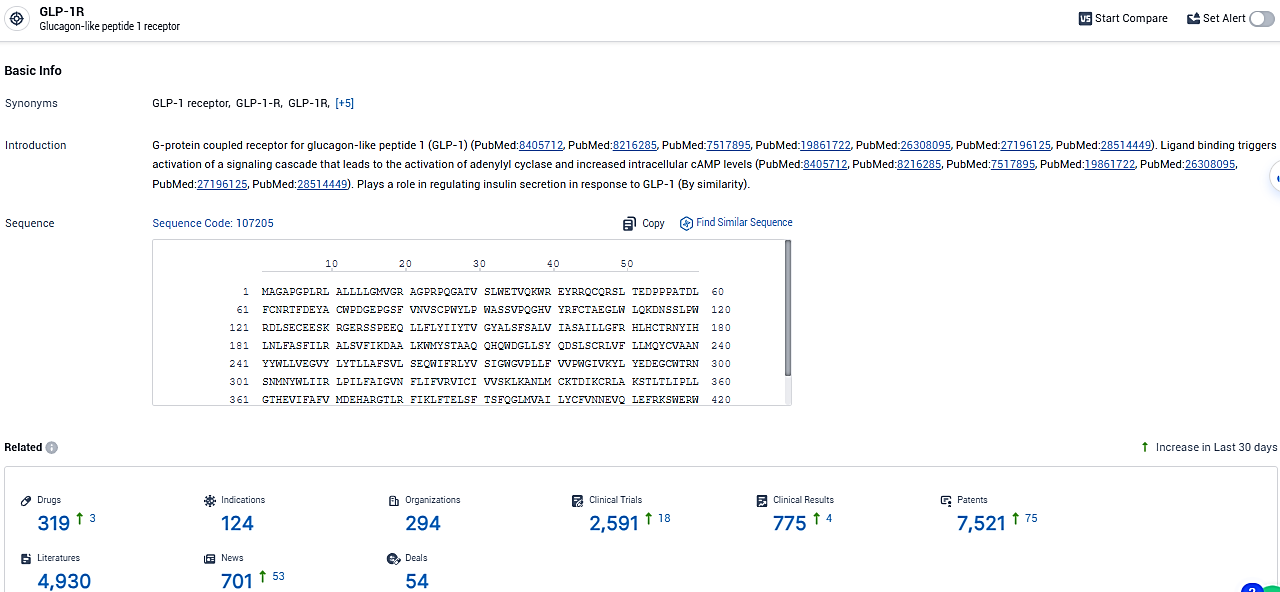
According to the data provided by the Synapse Database, As of January 30, 2024, there are 319 investigational drugs for the GLP-1R target, including 124 indications, 294 R&D institutions involved, with related clinical trials reaching 2591, and as many as 7521 patents.
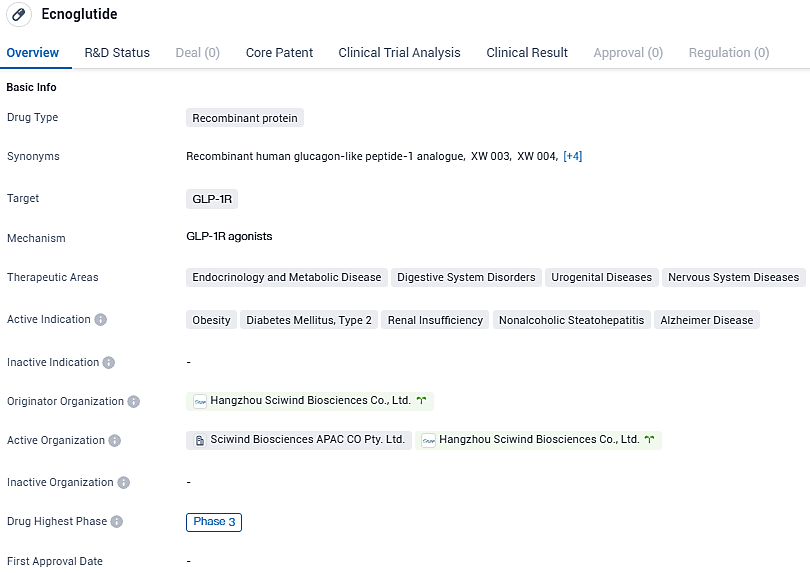
Ecnoglutide is a novel, cAMP signaling biased, long-acting GLP-1 analogue optimized for improved biological activity, cost-effective manufacturing, and once weekly dosing. It shows promise in treating various diseases, including obesity, type 2 diabetes mellitus, renal insufficiency, nonalcoholic steatohepatitis, and Alzheimer's disease. The drug's advancement to Phase 3 suggests positive results in earlier stages of testing, and if approved, Ecnoglutide could address significant unmet medical needs in these therapeutic areas.