siRNA and ASO: Exploring the Pillars of Small Nucleic Acid Therapeutics
siRNA (small interfering RNA) drugs and ASO (antisense oligonucleotide) drugs, as the two pillars of the small nucleic acid therapeutics field, share a core mechanism: modulating gene expression through sequence-specific complementary pairing with target RNA. This precise matching allows them to target specific mRNA molecules, thereby intervening in gene function at the post-transcriptional level and regulating intracellular protein synthesis processes.
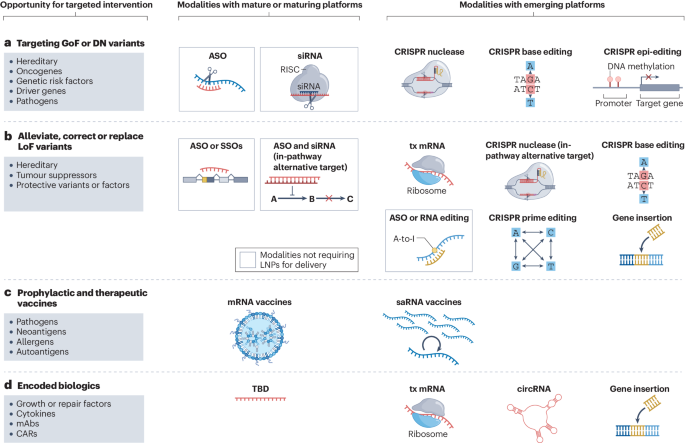
Despite their similar objectives, siRNA and ASO exhibit distinct differences in molecular structure and mechanisms of action. siRNA typically comprises double-stranded RNA molecules that interact with the RNA-induced silencing complex (RISC) to recognize and cleave specific mRNA targets. In contrast, ASOs are single-stranded DNA or RNA molecules that achieve gene silencing either by activating RNase H enzymes or influencing mRNA splicing. For delivery, siRNA often requires carriers such as liposomes or nanoparticles to enhance cellular uptake efficiency, whereas ASOs can utilize chemical modifications to improve stability and delivery in vivo. Additionally, ASOs can be designed to act on RNA inside and outside the nucleus, while siRNA predominantly operates in the cytoplasm.
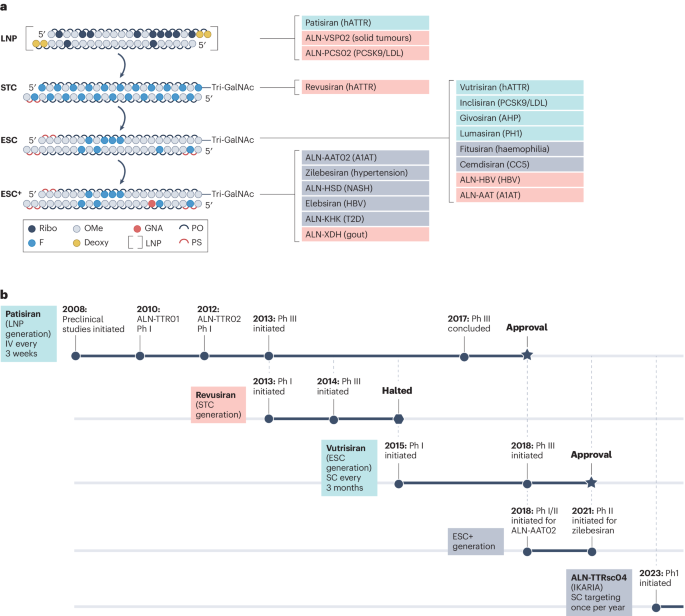
Using platforms like Patsnap Bio, researchers can rapidly access siRNA and ASO sequence information, enabling the design of sequences that efficiently recognize and cleave target mRNA (siRNA) or bind and induce degradation/alter splicing patterns (ASO). Such data are essential for developing highly specific and efficient small nucleic acid drugs. Patsnap Synapse further serves as a robust tool for tracking the latest developments in the small nucleic acid drug field. It facilitates insights into global R&D progress for siRNA and ASO drugs, competitor research, clinical trials, and market performance.
The mechanisms of action for ASO and siRNA drugs differ significantly:
1)ASO Drugs Activate RNase H Enzyme
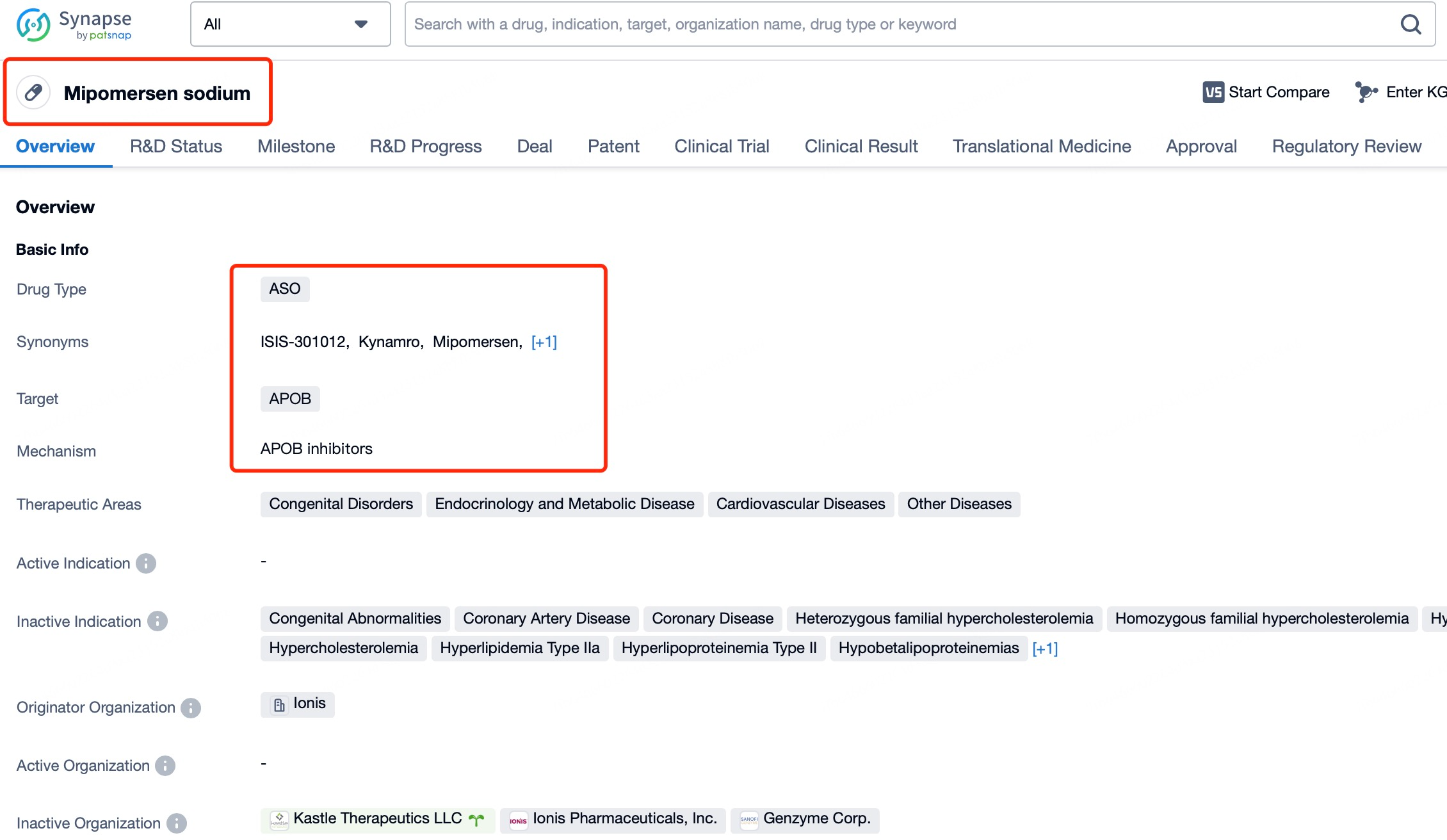
ASOs most commonly function by activating the intracellular RNase H enzyme, which identifies and cleaves the RNA strand in RNA-DNA hybrid molecules, leading to the degradation of target mRNA. A notable example is Kynamro (mipomersen), an ASO drug used to reduce low-density lipoprotein (LDL) cholesterol levels. Mipomersen specifically targets the mRNA in the liver responsible for producing ApoB-100, a key protein component of LDL particles. Elevated LDL cholesterol levels are a significant risk factor for cardiovascular diseases. By binding specifically to the mRNA sequence of ApoB-100, mipomersen triggers RNase H-mediated mRNA degradation, thereby reducing ApoB-100 protein synthesis. This results in decreased blood LDL cholesterol levels, mitigating cardiovascular disease risks.
To achieve precise gene silencing, mipomersen is chemically modified to enhance its stability, allowing effective cellular penetration and targeting intracellular mRNA. Additionally, its design minimizes immune recognition and clearance, prolonging its half-life in vivo and ensuring sustained therapeutic efficacy.
2)ASO Drugs Blocking Splicing
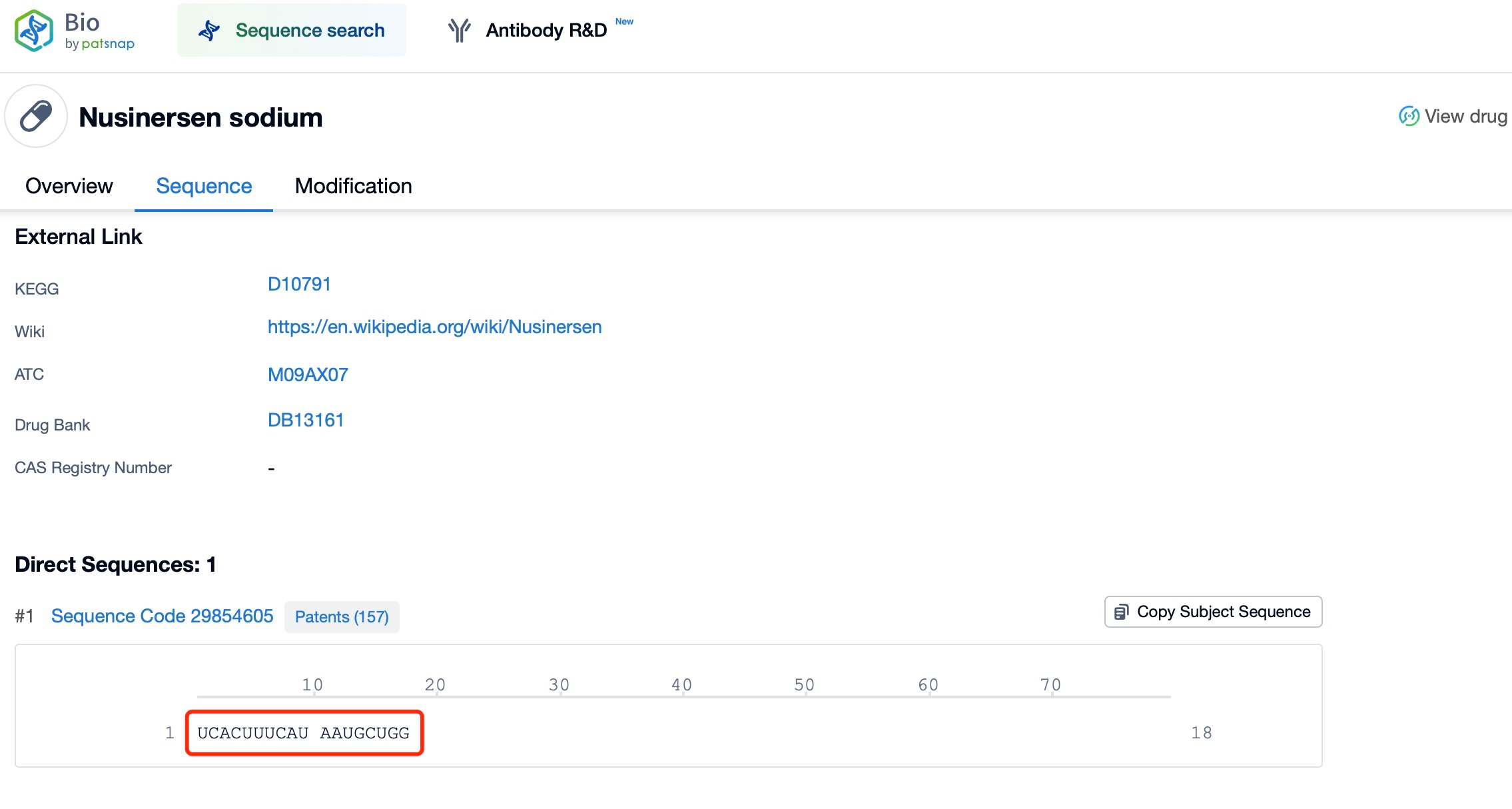
Some ASOs bind to precursor mRNA (pre-mRNA) and influence its normal splicing process. This interaction can exclude specific exons, altering mRNA splicing patterns and generating alternative mRNA isoforms. A prominent example is Spinraza (nusinersen), used to treat spinal muscular atrophy (SMA). Spinraza ensures the retention of exon 7 in the SMN2 gene's mRNA, producing a protein similar to that from the functional SMN1 gene.
In SMA patients, the SMN1 gene is usually mutated, resulting in insufficient functional SMN protein. However, the human genome includes a near-identical gene, SMN2, which produces limited functional SMN protein due to the frequent exclusion of exon 7 during splicing. Spinraza binds to SMN2 pre-mRNA, promoting exon 7 inclusion, enabling the production of full-length, functional SMN protein. This mechanism helps maintain the levels of SMN protein necessary for neuron survival and restores neuromuscular function in patients.
Spinraza exemplifies the potential of ASOs in precision medicine, particularly for treating genetic disorders caused by splicing errors. The successful development and application of such drugs demonstrate the feasibility of treating genetic diseases by modulating gene expression.
3)ASO Drugs Stabilizing mRNA
ASOs can stabilize mRNA by binding complementarily to specific sequences, protecting it from degradation and enhancing its stability and translational efficiency. By targeting the 3' untranslated region (3'UTR) or 5' untranslated region (5'UTR) of mRNA, ASOs prevent microRNA (miRNA) interactions with mRNA, thereby inhibiting the RNA-induced silencing complex (RISC) from degrading the mRNA. This action extends the lifespan of the mRNA within the cell.
In addition, ASOs can alter mRNA structure. For example, by binding to the 5'UTR, ASOs may induce structural changes that facilitate ribosome recognition of the start codon (AUG), thereby increasing translation efficiency. Furthermore, ASOs can suppress inhibitory elements within the 5'UTR that negatively impact translation, enhancing overall protein synthesis.
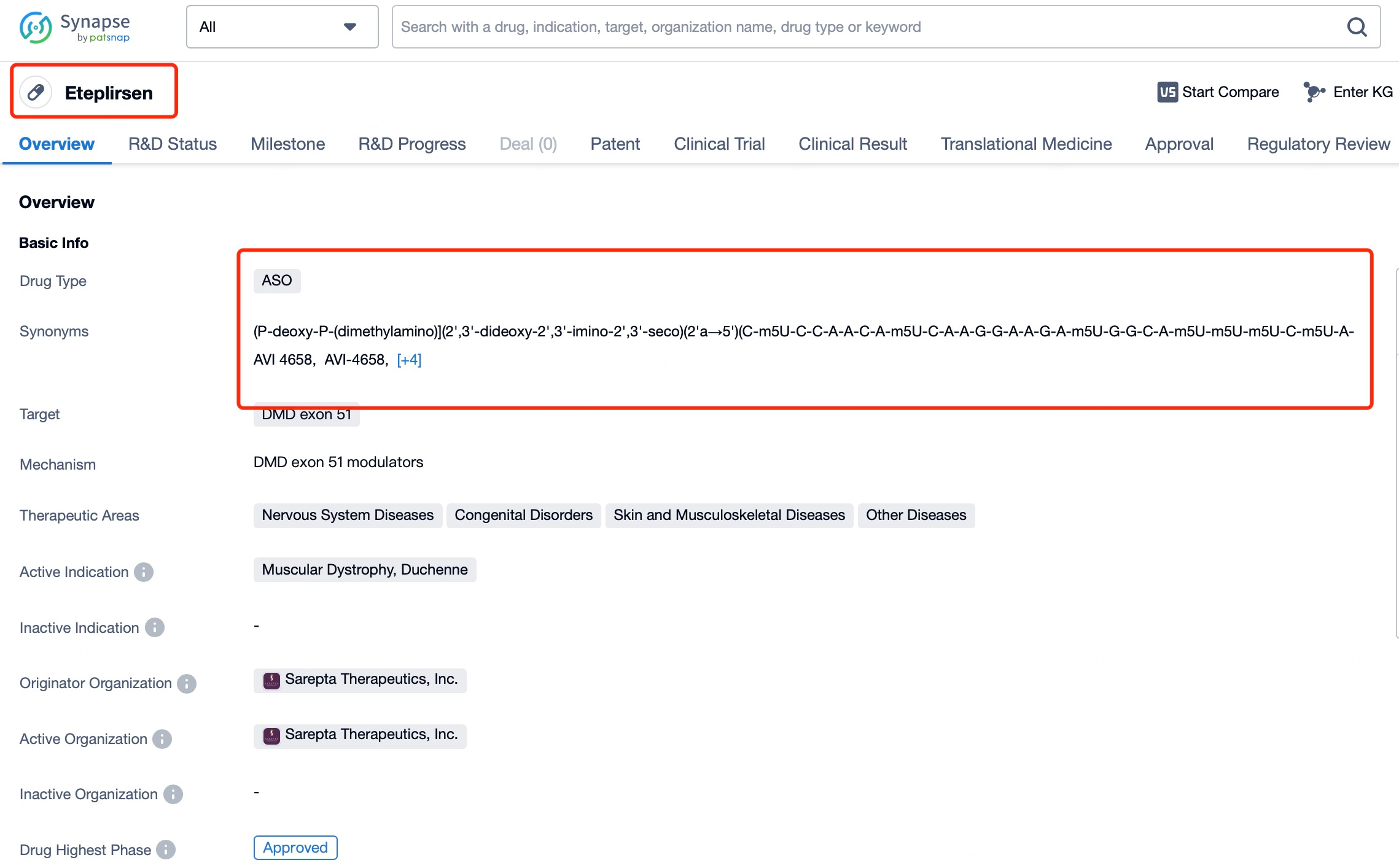
A practical example is Exondys 51 (eteplirsen), a drug used to treat Duchenne muscular dystrophy (DMD). Eteplirsen works by binding to the pre-mRNA of the DMD gene, directing the splicing machinery to skip exon 51 during mRNA processing. This mechanism bypasses the mutation, enabling the production of a shortened but partially functional dystrophin protein.
In DMD patients, genetic mutations often disrupt normal splicing, leading to defective or nonfunctional dystrophin proteins. By targeting the specific sequence on pre-mRNA, eteplirsen facilitates the exclusion of exon 51, allowing for the synthesis of a dystrophin protein that retains partial functionality. This modified splicing approach avoids the effects of the mutation, permitting the expression of a functional protein. Although the resulting protein may be less abundant and less effective than the normal dystrophin, it provides symptomatic relief and stabilization for DMD patients.
4)Mechanism of siRNA
Small interfering RNA (siRNA) mediates precise gene silencing by interacting with the RNA-induced silencing complex (RISC). RISC is a protein complex, with Argonaute 2 (Ago2) as a pivotal component possessing endonuclease activity. Once the siRNA duplex is integrated into RISC, it guides the complex to cytoplasmic mRNA molecules that are complementary to the siRNA sequence. Upon target mRNA recognition, Ago2 catalyzes the cleavage of the mRNA strand, resulting in its degradation. This degradation prevents the mRNA from being translated into protein, effectively silencing the target gene. This process, termed gene silencing, eliminates specific mRNA molecules to reduce the corresponding protein levels, achieving therapeutic outcomes.
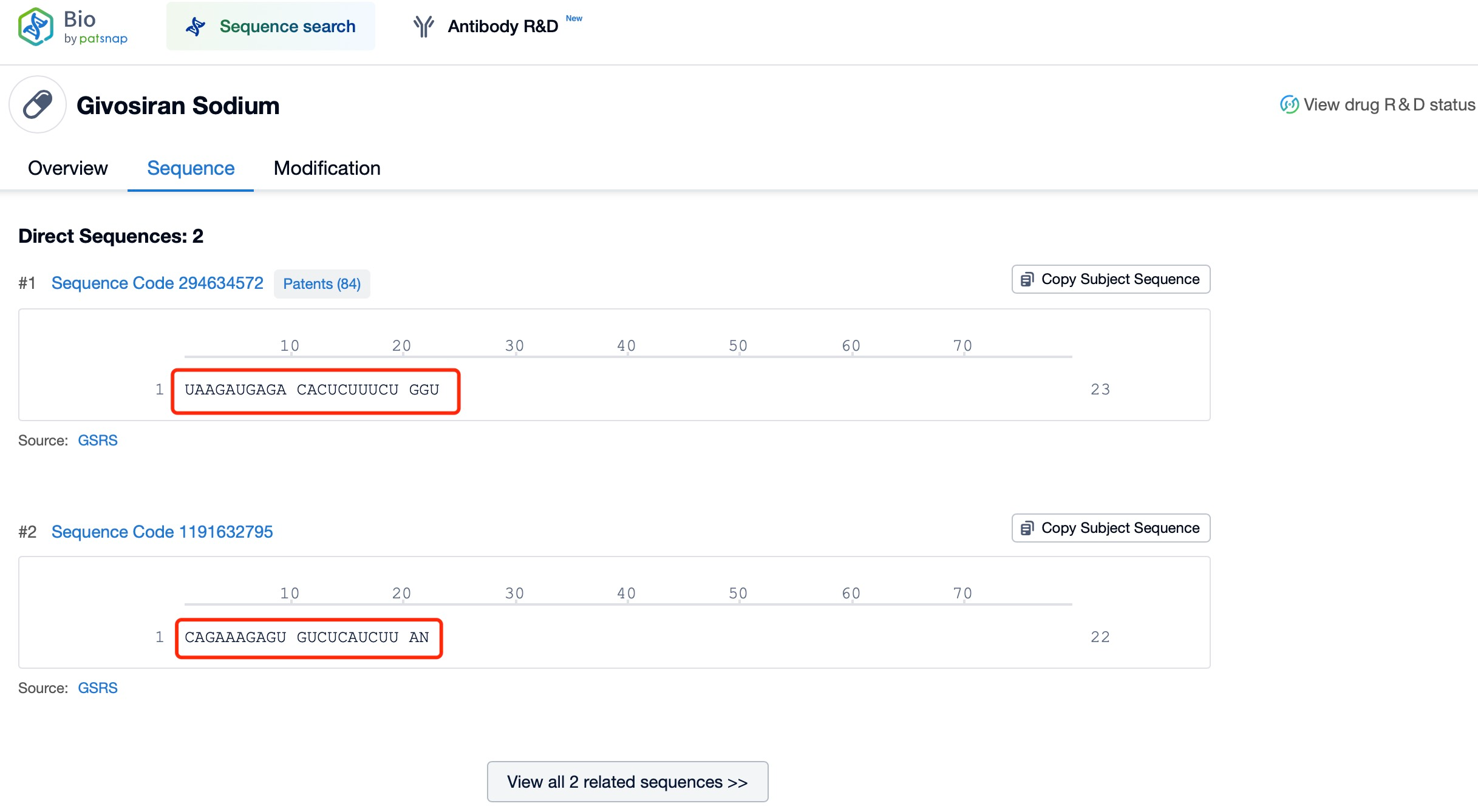
For example, Givosiran (trade name: Givlaari®), developed by Alnylam Pharmaceuticals, is an siRNA drug designed to treat adults with acute hepatic porphyria (AHP). Givosiran specifically degrades the mRNA encoding ALAS1, a protein involved in the production of toxic heme intermediates. Utilizing siRNA’s gene silencing mechanism, Givosiran binds to RISC and activates Ago2’s endonuclease activity, which cleaves the complementary mRNA, reducing ALAS1 protein production. This targeted mechanism mitigates symptoms of AHP, exemplifying RNA interference (RNAi) technology’s potential in treating rare diseases.
Summary:
siRNA and ASOs are critical components of modern gene therapy, each regulating gene expression through unique mechanisms. siRNA operates by activating the RNA-induced silencing complex (RISC) and utilizing the endonuclease activity of the Argonaute 2 (Ago2) protein to recognize and cleave complementary mRNA, leading to mRNA degradation and suppression of specific protein synthesis. This mechanism is particularly active in the cytoplasm, making siRNA highly effective in treating liver diseases. On the other hand, ASOs modulate gene expression through multiple mechanisms, including activating RNase H to degrade mRNA, altering pre-mRNA splicing patterns, and preventing the binding of translation initiation factors to mRNA, thereby inhibiting protein synthesis or modifying protein structure. The complementarity of these two techniques suggests that the combined use of siRNA and ASOs may provide more comprehensive therapeutic solutions for complex disease models.
If you are interested in understanding the market dynamics and competitive landscape in ASO and siRNA, click on Patsnap Synapse.
To learn more about bio sequences and modifications, click on Patsnap Bio.
Refrence:
Androsavich, J. R. Frameworks for transformational breakthroughs in RNA-based medicines. Nat Rev Drug Discov 23, 421-444 (2024). https://doi.org:10.1038/s41573-024-00943-2