STADA and Alvotech Launch Uzpruvo, Europe's First Approved Ustekinumab Biosimilar to Stelara
STADA and Alvotech have introduced Uzpruvo, the inaugural biosimilar to Stelara approved in Europe, in numerous European nations. This encompasses the principal markets in the area, where they have obtained pricing and reimbursement approvals necessary for market penetration.
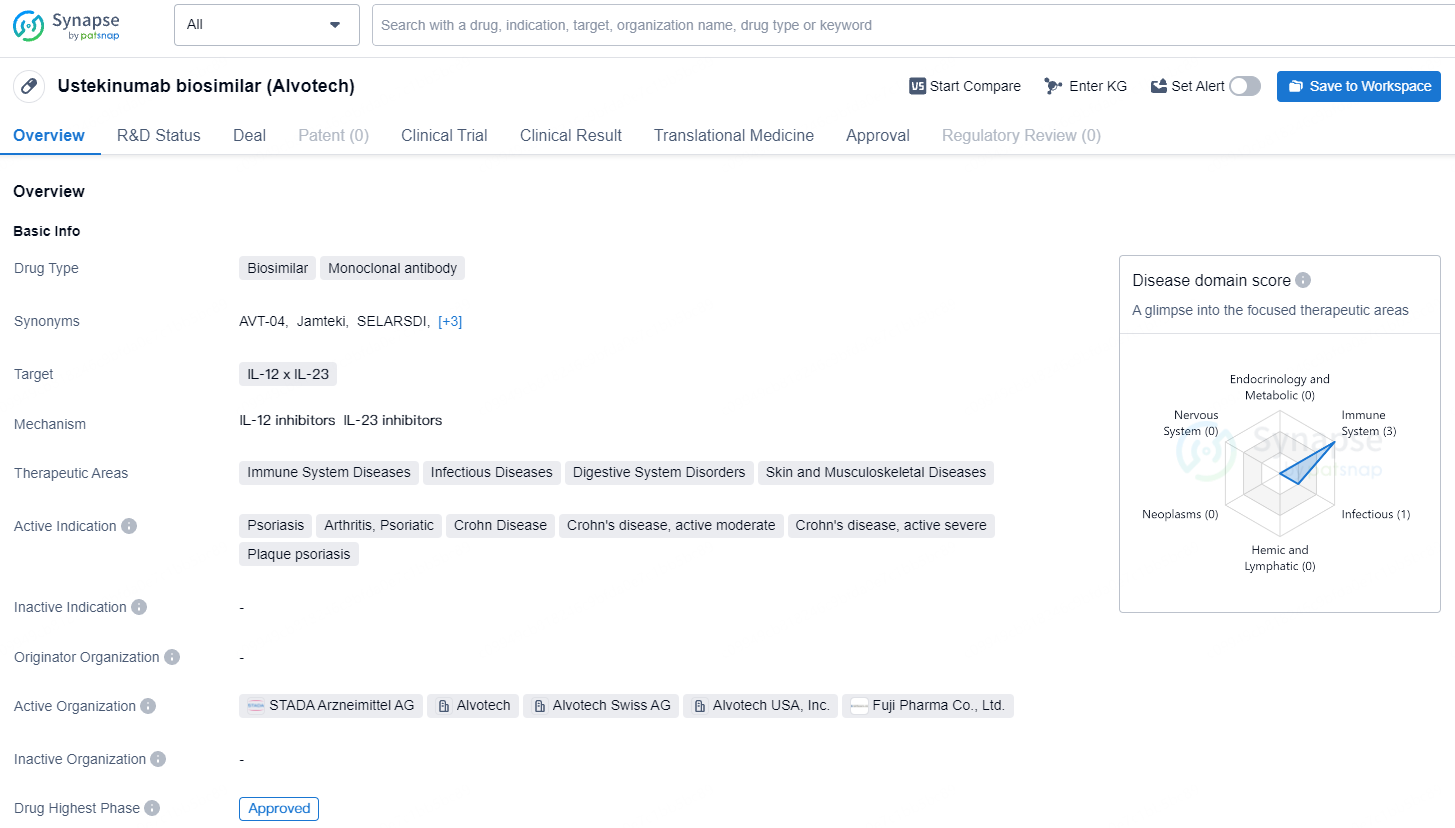
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
 The groundbreaking launch follows the expiration of exclusivity rights related to the European reference molecule patent, providing expanded access to a critical medicine at the earliest possible time for patients, physicians, and payers. This drug is used in specific indications within the fields of gastroenterology, dermatology, and rheumatology.
The groundbreaking launch follows the expiration of exclusivity rights related to the European reference molecule patent, providing expanded access to a critical medicine at the earliest possible time for patients, physicians, and payers. This drug is used in specific indications within the fields of gastroenterology, dermatology, and rheumatology.
"By introducing Uzpruvo at the first available opportunity in Europe's major pharmaceutical markets, we are enhancing access through increased competition," stated STADA CEO Peter Goldschmidt. "Our aim to broaden patient access to this transformative biological treatment aligns with STADA’s mission of Caring for People’s Health as a Trusted Partner."
"We are excited about the launch of Uzpruvo in Europe and being the first to market," said Robert Wessman, Chief Executive Officer of Alvotech. "This achievement highlights the strength of our platform, the value of our collaboration with STADA, and our shared commitment to the significance of biosimilars."
In January 2024, Uzpruvo became the first ustekinumab biosimilar authorized by the European Commission, demonstrating equivalent efficacy, safety, pharmacokinetics, and immunogenicity to the Stelara reference product. Uzpruvo is approved for use in adults with Crohn’s disease and psoriatic arthritis, as well as plaque psoriasis in adults and children aged 6 years and older. It is not approved for ulcerative colitis, as the original product retains exclusivity for this indication.
Uzpruvo is available in a pre-filled syringe format that includes a thinner needle compared to the reference product and is latex-free to reduce the risk of allergic reactions. Uzpruvo is developed, manufactured, and packaged entirely within Europe, and boasts a 36-month shelf life.
"With matching safety, efficacy, and immunogenicity, Uzpruvo offers clinicians a straightforward option for transitioning their patients. The thinner needle and latex-free syringe provide additional benefits," commented Bryan Kim, STADA’s Global Specialty Head. "Physicians and patients can trust that STADA has over 15 years of experience in improving patient access through high-quality biosimilars in Europe, having launched our first biosimilar in 2008."
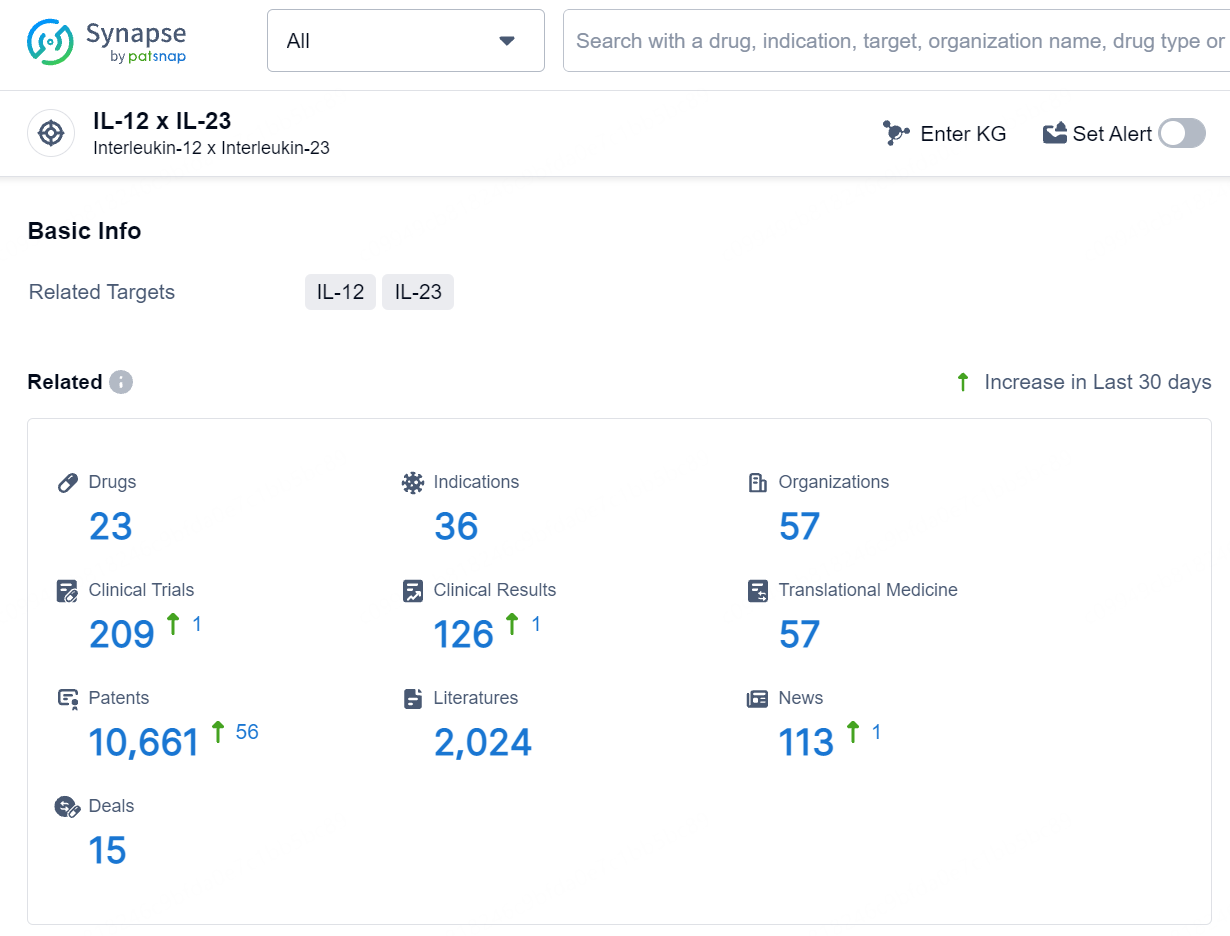
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of July 24, 2024, there are 23 investigational drugs for the IL-12 and IL-23 targets, including 36 indications, 57 R&D institutions involved, with related clinical trials reaching 209, and as many as 10661 patents.
Ustekinumab biosimilar is a monoclonal antibody drug that falls under the category of biosimilars. It targets IL-12 and IL-23 and is developed to treat a range of conditions related to the immune system, infectious diseases, digestive system disorders, and skin and musculoskeletal diseases. The development and commercialization of biosimilar drugs are expected to play a key role in improving access to essential treatments and driving market competition, ultimately benefiting both patients and healthcare systems worldwide.