Stavudine: Detailed Review of its Transformative R&D Success
Stavudine's R&D Progress
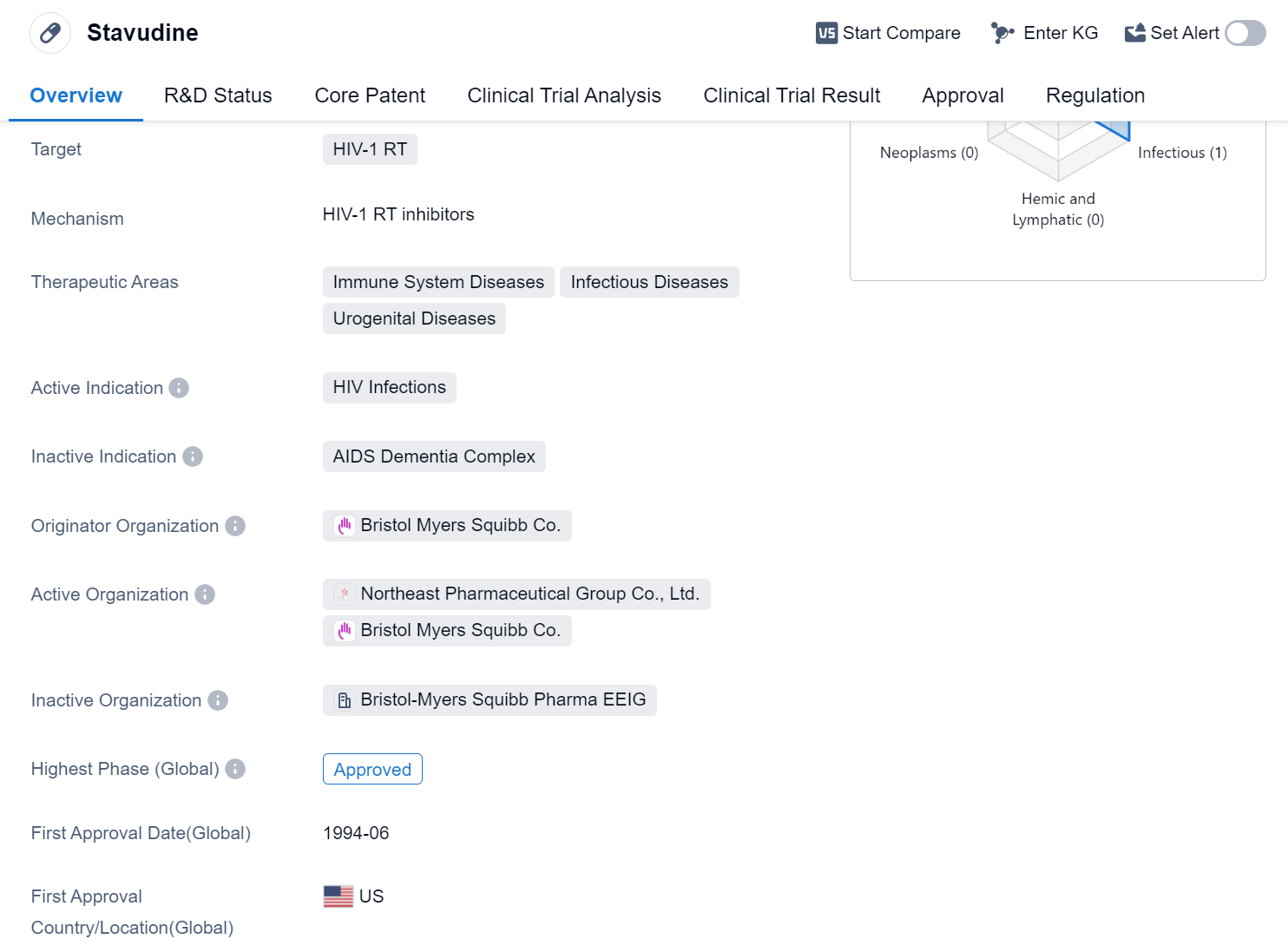
Stavudine is a small molecule drug that is primarily used for the treatment of HIV infections. It specifically targets the HIV-1 RT enzyme, which plays a crucial role in the replication of the virus. The drug is classified under the therapeutic areas of immune system diseases, infectious diseases, and urogenital diseases, highlighting its importance in managing HIV infections.
Stavudine was first approved for use in the United States in June 1994 by the originator organization, Bristol Myers Squibb Co. It has since received approval in other countries as well, making it a globally recognized drug for the treatment of HIV infections. In China, it has also obtained approval, indicating its availability and use in the Chinese market.
The regulatory status of Stavudine is notable, as it has been granted accelerated approval and orphan drug designation. Accelerated approval is a regulatory pathway that allows for the expedited approval of drugs that address unmet medical needs, such as HIV infections. Orphan drug designation is granted to drugs that are intended to treat rare diseases or conditions, further emphasizing the importance of Stavudine in managing HIV infections.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Stavudine: HIV-1 RT inhibitors
HIV-1 RT inhibitors are a class of drugs used in the treatment of HIV infection. HIV-1 refers to the most common strain of the human immunodeficiency virus (HIV), which is responsible for the majority of HIV infections worldwide. RT stands for reverse transcriptase, an enzyme that plays a crucial role in the replication of the virus.
HIV-1 RT inhibitors specifically target the reverse transcriptase enzyme, inhibiting its activity and preventing the virus from replicating. These inhibitors can be further classified into two main types: nucleoside/nucleotide RT inhibitors (NRTIs) and non-nucleoside RT inhibitors (NNRTIs).
NRTIs are analogs of the building blocks of DNA and RNA. When incorporated into the growing viral DNA chain, they terminate the chain and prevent further replication. NNRTIs, on the other hand, bind to a specific site on the reverse transcriptase enzyme, causing a conformational change that inhibits its function.
By targeting HIV-1 RT, these inhibitors help to reduce the viral load in the body, slow down the progression of HIV infection, and improve the immune function of individuals living with HIV. They are typically used in combination with other antiretroviral drugs as part of highly active antiretroviral therapy (HAART) to effectively manage HIV infection and prevent the development of acquired immunodeficiency syndrome (AIDS).
Drug Target R&D Trends for Stavudine
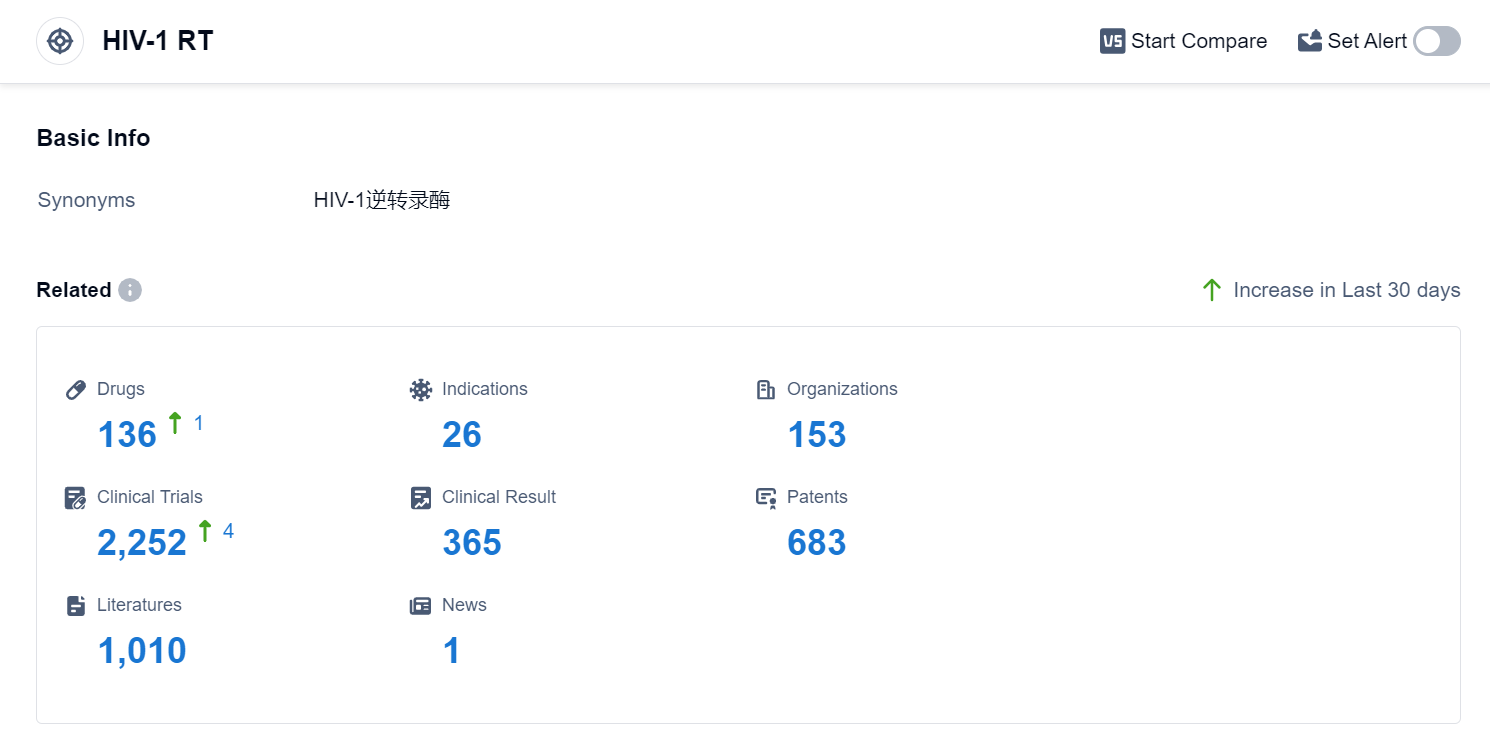
According to Patsnap Synapse, as of 4 Sep 2023, there are a total of 136 HIV-1 RT drugs worldwide, from 153 organizations, covering 26 indications, and conducting 2252 clinical trials.
Based on the analysis of the data provided, the current competitive landscape for the target HIV-1 RT in the pharmaceutical industry is dominated by companies such as Gilead Sciences, Inc., GSK Plc, and Johnson & Johnson. These companies have made significant progress in the research and development of drugs targeting HIV-1 RT. The approved drugs under this target primarily focus on the treatment of HIV Infections and HIV-1 infection. Small molecule drugs are the most rapidly progressing drug type, indicating intense competition in the development of innovative drugs. The leading countries/locations in drug development for HIV-1 RT include the European Union, United States, China, and Japan. China has shown notable progress in drug development for this target. Overall, the future development of target HIV-1 RT holds promise, with ongoing research and development efforts focused on addressing various indications and advancing drug types.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Stavudine has a long history of use in the treatment of HIV infections, with its first approval dating back to 1994. Its approval in multiple countries, including the United States and China, highlights its global recognition and availability. The drug's specific targeting of the HIV-1 RT enzyme makes it a valuable tool in managing the replication of the virus. Additionally, its regulatory status of accelerated approval and orphan drug designation further underscores its significance in addressing unmet medical needs in the field of HIV treatment.