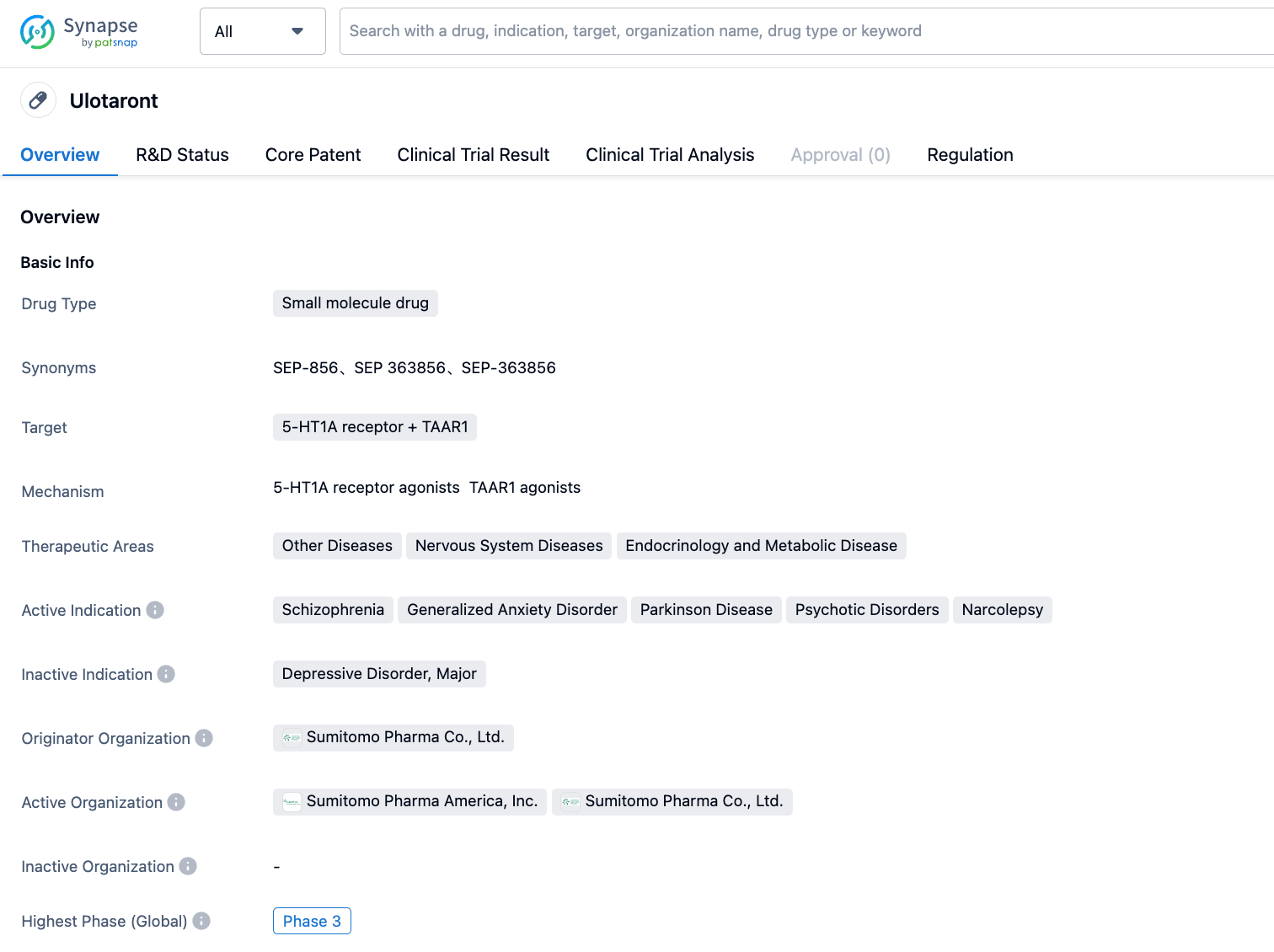
Sumitomo Dainippon Pharma/Otsuka Pharmaceutical announced that two Phase III studies of Ulotaront did not meet the primary endpoints
On July 31, 2023, Sumitomo Dainippon Pharma and Otsuka Pharmaceutical jointly announced that their two Phase III studies, DIAMOND 1 and DIAMOND 2, for ulotaront (SEP-363856) in the treatment of acute schizophrenia did not meet the primary endpoints.
Ulotaront is a dual agonist of Trace Amine-Associated Receptor 1 (TAAR1) and 5-Hydroxytryptamine 1A (5-HT1A) developed by Sumitomo subsidiary Sunovion. It is currently under clinical research for multiple indications including schizophrenia, generalized anxiety disorder, and major depressive disorder. In May 2019, it was granted Breakthrough Therapy Designation by the FDA for the treatment of schizophrenia. TAAR is a G protein-coupled receptor widely distributed in various tissues and can be activated not only by trace amines but also by monoamines such as dopamine or serotonin, with TAAR1 being the most widely distributed TAAR in the brain. Some schizophrenic patients reportedly have TAAR1 gene mutations. In September 2021, Otsuka Pharmaceutical spent nearly $900 million ($270 million upfront payment and $620 million in Biobucks) to acquire the co-development and commercialization rights for ulotaront and three other psychiatric disease candidate drugs from Sumitomo Dainippon Pharma.
The DIAMOND 1 study was a multi-center, random, double-blind, parallel group, fixed-dose Phase III clinical trial aimed at assessing the efficacy, safety, and tolerability of ulotaront (50mg/day and 75mg/day) versus placebo in adult patients with acute schizophrenia over a period of 6 weeks, involving a total of 435 subjects. The results showed that the Positive and Negative Syndrome Scale (PANSS) scores of all three study groups decreased over time, but in the main endpoint of change in PANSS score at 6 weeks from baseline, both treatment groups were not superior to the placebo group. DIAMOND 2 was a similar study evaluating ulotaront (75mg/day and 100mg/day) with 464 subjects. The results showed that both treatment groups did not achieve statistically significant improvements in the primary endpoint compared to the placebo.
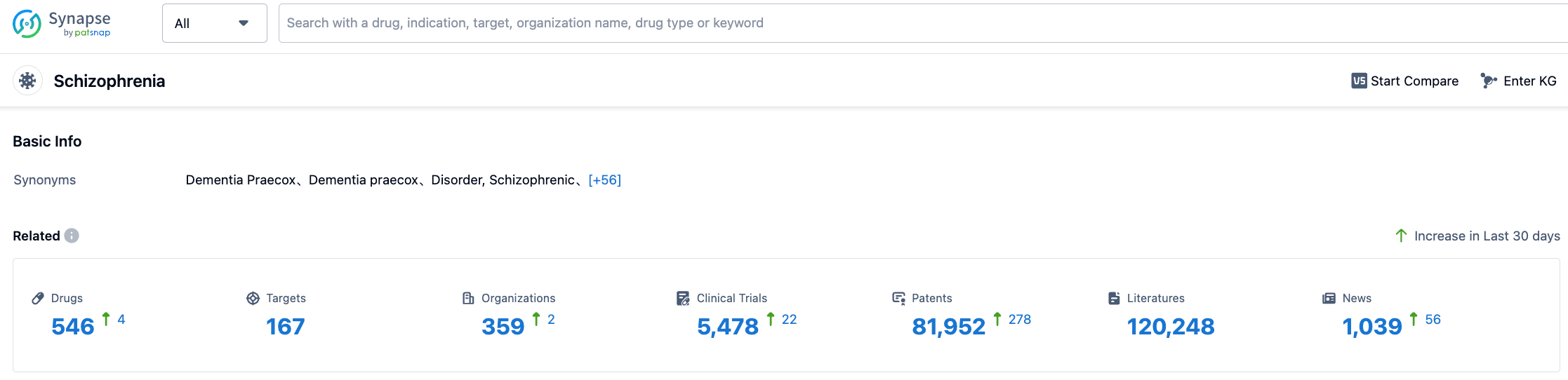
According to data disclosed in Synapse, as of August 2, 2023, 546 new drugs were under development for schizophrenia indication, involving 167 target points, 359 research institutes, 5477 related clinical trials, and 81942 patents.The development of new drugs for schizophrenia is challenging and has a high rate of failure. The medical community is actively awaiting the emergence of new effective therapies.