The development of lncRNA drugs is key to regulating the treatment of tumors and cardiovascular diseases
Long noncoding RNAs (LncRNA) are a type of transcript that is over 200nt in length and does not code for proteins. Initially, this type of RNA was considered “noise” in genome transcription. It is estimated that about 93% of transcripts are lncRNAs, which are generally located in the cell nucleus and cytoplasm. However, the gene transcription level of lncRNA is usually lower than that of protein-coding genes, and they are poorly conserved, subjected to low evolutionary pressure, but their promoter sequences are usually conserved. Compared with small molecule RNAs, lncRNAs have longer sequences and more complex spatial structures, and the mechanisms involved in expression regulation are more diverse and complex. RNA capture sequencing technology (CLS) can analyze the genome with higher accuracy, thereby deeply understanding lncRNA. Relevant studies have shown that human cardiovascular diseases are directly related to the abnormal expression of lncRNAs. lncRNAs regulate myocardial infarction, atherosclerosis, heart failure, myocardial hypertrophy, ischemic cardiomyopathy, and lipids in cardiovascular diseases.
Although research on circulating lncRNA is still in its early stages, there is a growing worldwide interest in lncRNA, and the emergence of new technologies to improve detection and specificity is undeniable. This increases the potential for clinical applications and the chance of one day discovering reliable blood biomarkers that would allow for early and accurate detection of any type of cancer and cardiovascular disease.
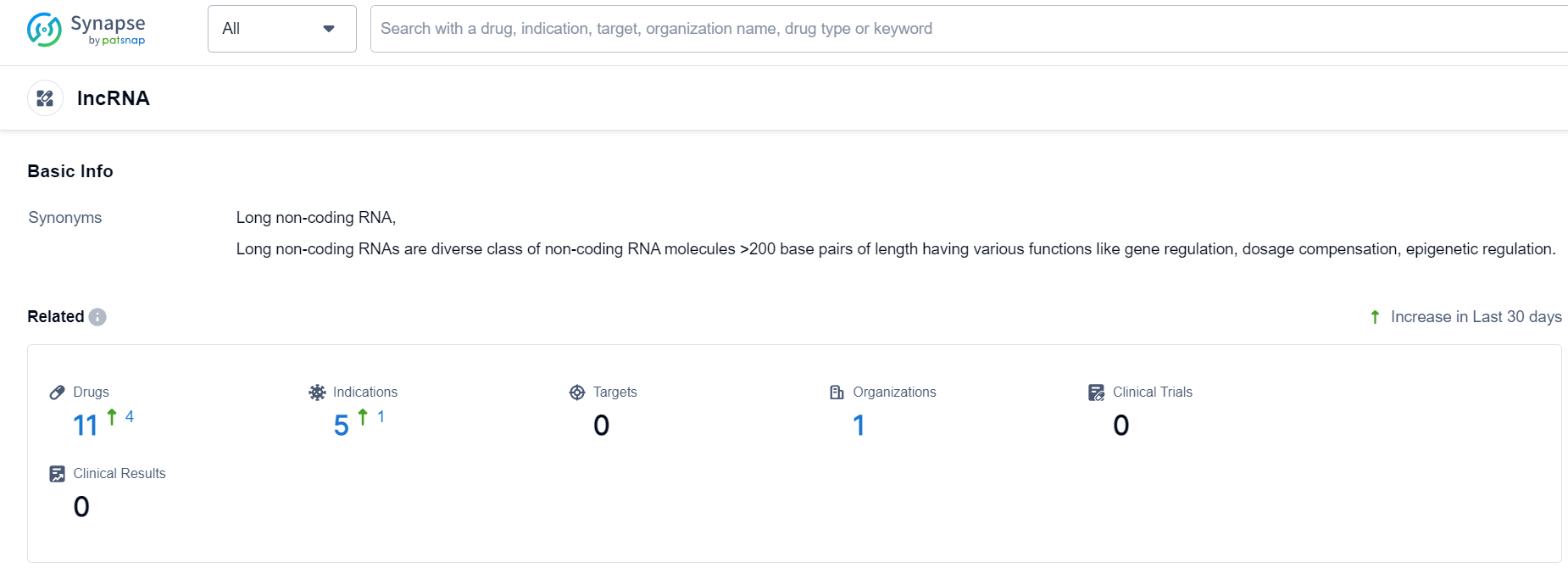
LncRNA Competitive Landscape
According to Patsnap Synapse, as of 24 Sep 2023, there are a total of 11 lncRNA drugs worldwide, from 1 organizations, covering 0 targets, 5 indications, and conducting 0 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, targets, organizations, clinical trials, and clinical results related to this drug type.
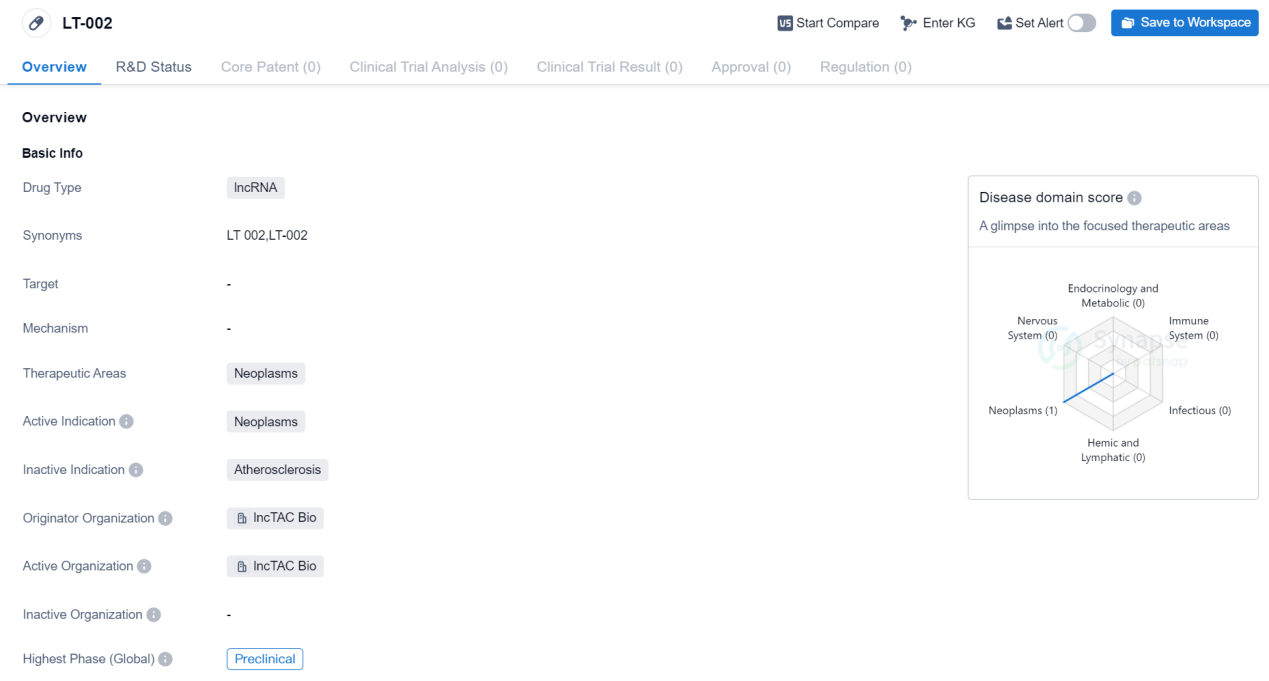
PreClinical lncRNA Medicinal: LT-002
The drug LT-002 is a type of lncRNA, which stands for long non-coding RNA. It is being developed for the treatment of neoplasms, which are abnormal growths of tissue commonly known as tumors. The drug is specifically targeting neoplasms and is intended to provide therapeutic benefits in this area.
The drug LT-002 is being developed by lncTAC Bio, which is the originator organization responsible for its development. As of the latest available information, the drug is in the preclinical phase, which means it is still undergoing laboratory testing and has not yet progressed to human clinical trials.
The drug is still in the early stages of development and has not yet advanced to clinical trials in any country. Preclinical studies involve testing the drug in laboratory settings and animal models to assess its safety, efficacy, and potential side effects before it can be tested in humans.
Based on the information provided, it can be concluded that LT-002 is a novel lncRNA drug being developed by lncTAC Bio for the treatment of neoplasms. However, since the drug is still in the preclinical phase, it is too early to determine its potential effectiveness or its future prospects in terms of clinical development and commercialization.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In summary, LT-002 is a lncRNA drug being developed by lncTAC Bio for the treatment of neoplasms. It is currently in the preclinical phase of development globally. Further research and development will be required to assess its safety and efficacy before it can progress to clinical trials and potentially become a viable treatment option for neoplasms.