The European Commission has approved Blueprint Medicines' AYVAKYT® (avapritinib) as the sole treatment for indolent systemic mastocytosis
Blueprint Medicines Corporation has declared that the European Commission has sanctioned the use of AYVAKYT® (avapritinib) as a therapeutic option for adult individuals experiencing moderate to severe symptoms of indolent systemic mastocytosis, which have not been effectively managed with existing symptomatic treatments. AYVAKYT® stands as the novel and sole therapy ratified for the management of indolent systemic mastocytosis in the European territory.
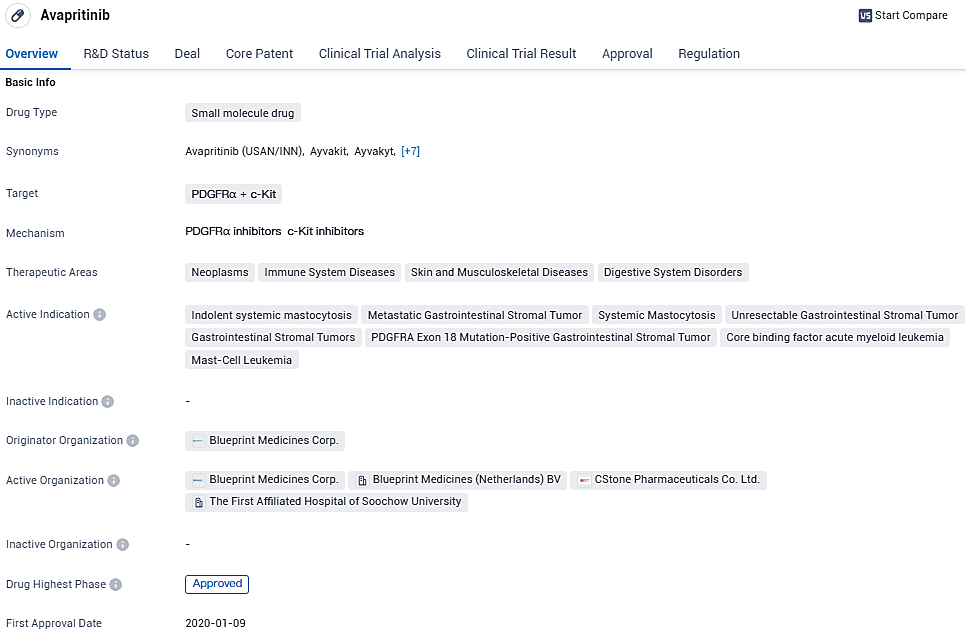
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Systemic mastocytosis, classified as an uncommon blood-related illness, can cause various severe symptoms that drastically affect patients' well-being. Most individuals affected by SM are diagnosed with ISM, with an estimated 40,000 cases in the European Union. AYVAKYT has been engineered to powerfully and precisely inhibit KIT D816V, the chief cause of this medical condition.
"The greenlighting of this medication is a pivotal move in setting a new international care benchmark for ISM patients and is founded on extended collective efforts with the SM community," noted Georg Pirmin Meyer, M.D., the Senior Vice President, International at Blueprint Medicines.
Dr. Georg Pirmin Meyer added, "For the inaugural occasion, ISM sufferers in Europe have an endorsed treatment, heralding a groundbreaking phase in combating this illness. AYVAKYT stands as the premiere therapy sanctioned for both ISM and advanced SM, with our dedicated team eager to make this revolutionary treatment accessible to individuals affected by varying stages of the disorder."
The endorsement stems from a favorable view by the Committee for Medicinal Products for Human Use. This authorization by the European Commission relies on the outcomes from the PIONEER trial, a comprehensive, placebo-controlled, double-masked study uniquely conducted among ISM patients. AYVAKYT has demonstrated significant enhancements in comparison to placebo concerning the primary efficacy goals and all crucial secondary benchmarks, which include total symptoms and quantifiers of mast cell presence.
With the intention to start offering this treatment in Europe, Blueprint Medicines is preparing to launch AYVAKYT commercially first in Germany. Subsequent introductions in other markets will follow, contingent on each country's health technology valuation and cost coverage mechanisms.
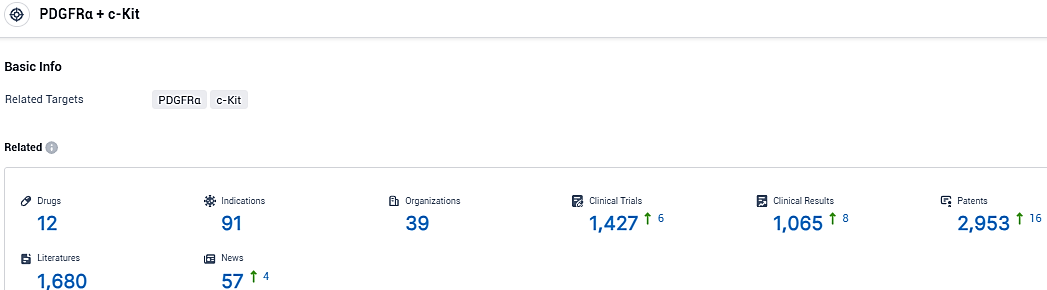
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of December 19, 2023, there are 12 investigational drugs for the PDGFRα and c-Kit target, including 91 indications, 39 R&D institutions involved, with related clinical trials reaching 1427, and as many as 2953 patents.
AYVAKYT® (avapritinib) is a kinase inhibitor approved by the European Commission for the treatment of three indications: adults with indolent systemic mastocytosis, adults with aggressive systemic mastocytosis, systemic mastocytosis with an associated hematological neoplasm or mast cell leukemia, after at least one systemic therapy, and adults with unresectable or metastatic gastrointestinal stromal tumors harboring the PDGFRA D842V mutation.