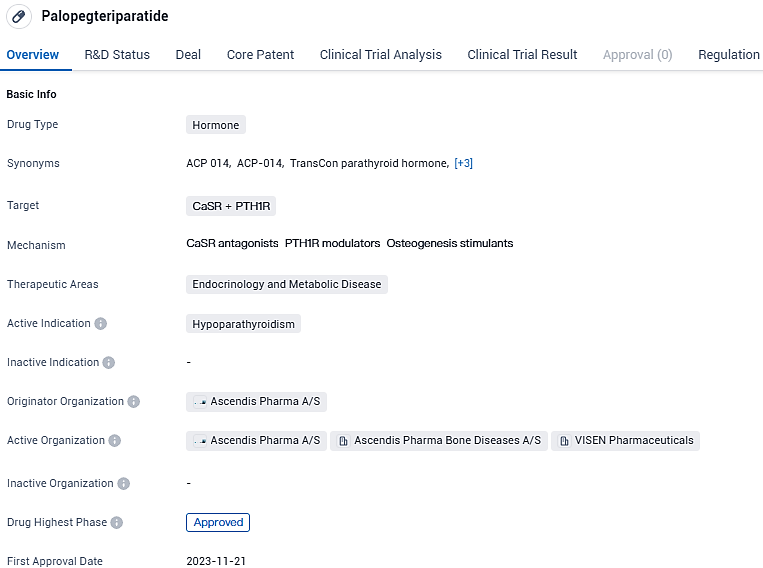
The FDA is evaluating the revised NDA for TransCon PTH (Palopegteriparatide) for adult hypoparathyroidism
Ascendis Pharma A/S has made a public statement that the U.S. Food and Drug Administration (FDA) has acknowledged and is in the process of evaluating the resubmitted New Drug Application of the company's TransCon PTH(palopegteriparatide), which is aimed at addressing the medical condition of hypoparathyroidism in adult individuals.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The office recognized the renewed submission as a comprehensive, tier 2 reply and designated a target date of May 14, 2024, under the guidelines of the Prescription Drug User Fee Act. In the United States, TransCon PTH (palopegteriparatide) remains experimental as a prodrug version of parathyroid hormone aimed at treating adult individuals living with hypoparathyroidism.
"The FDA's continuing evaluation of TransCon PTH is an encouraging step, and we anticipate further collaboration with the office throughout this process," stated Jan Mikkelsen, the President and Chief Executive Officer of Ascendis Pharma. "We are dedicated to promptly providing TransCon PTH to the American citizens, especially considering the critical needs yet to be met within the hypoparathyroidism population."
Ascendis Pharma is employing its inventive TransCon technology framework to establish itself as a front-runner and fully comprehensive biopharmaceutical entity, with a dedication to significantly improve patient welfare. The maiden authorization of Palopegteriparatide is anticipated by November 2023, with the European Union poised to be the initial district to consent to the drug. It implies the medication is presently under the scrutiny of regulatory oversight to confirm its safety and effectiveness for patient use.
Palopegteriparatide has achieved expedited inspection status, underscoring its urgency as a remedy capable of satisfying a critical healthcare void. Furthermore, it has received recognition as an orphan drug, signifying its aim to counteract a less common medical condition.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
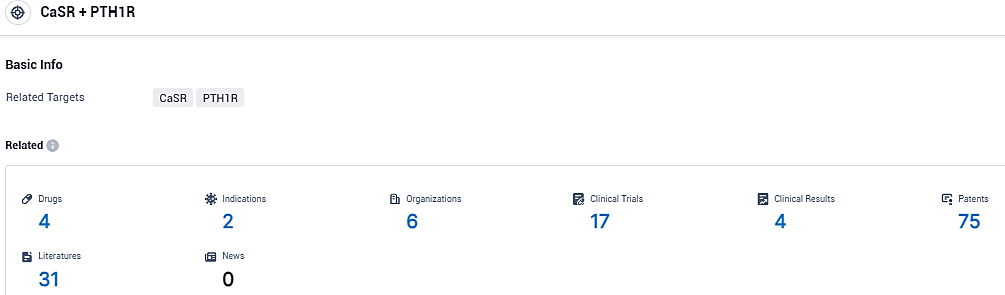
According to the data provided by the Synapse Database, As of December 19, 2023, there are 4 investigational drugs for the CaSR and PTH1R target, including 2 indications, 6 R&D institutions involved, with related clinical trials reaching 17, and as many as 75 patents.
Palopegteriparatide appears to be a promising drug in the field of endocrinology and metabolic disease, specifically for the treatment of hypoparathyroidism. Its development has progressed to the highest phase globally, with approval expected in the near future. The drug's regulatory status as a priority review and orphan drug further highlights its potential significance in addressing unmet medical needs.