Reflections on the FDA Hold of Medilink Therapeutics' HER3 ADC YL202/BNT326
On June 17, 2024, BioNTech announced that it had been informed by its partner Medilink Therapeutics that the FDA had partially suspended the Phase I clinical trial (YL202-INT-101-01) of their HER3 ADC YL202/BNT326, halting the enrollment of new patients in the United States.
In documents submitted to the U.S. Securities and Exchange Commission (SEC), BioNTech revealed that the FDA's partial suspension of the clinical trial was due to concerns that BNT326/YL202, at higher doses, might pose an unreasonable and significant risk of illness or injury to human subjects.

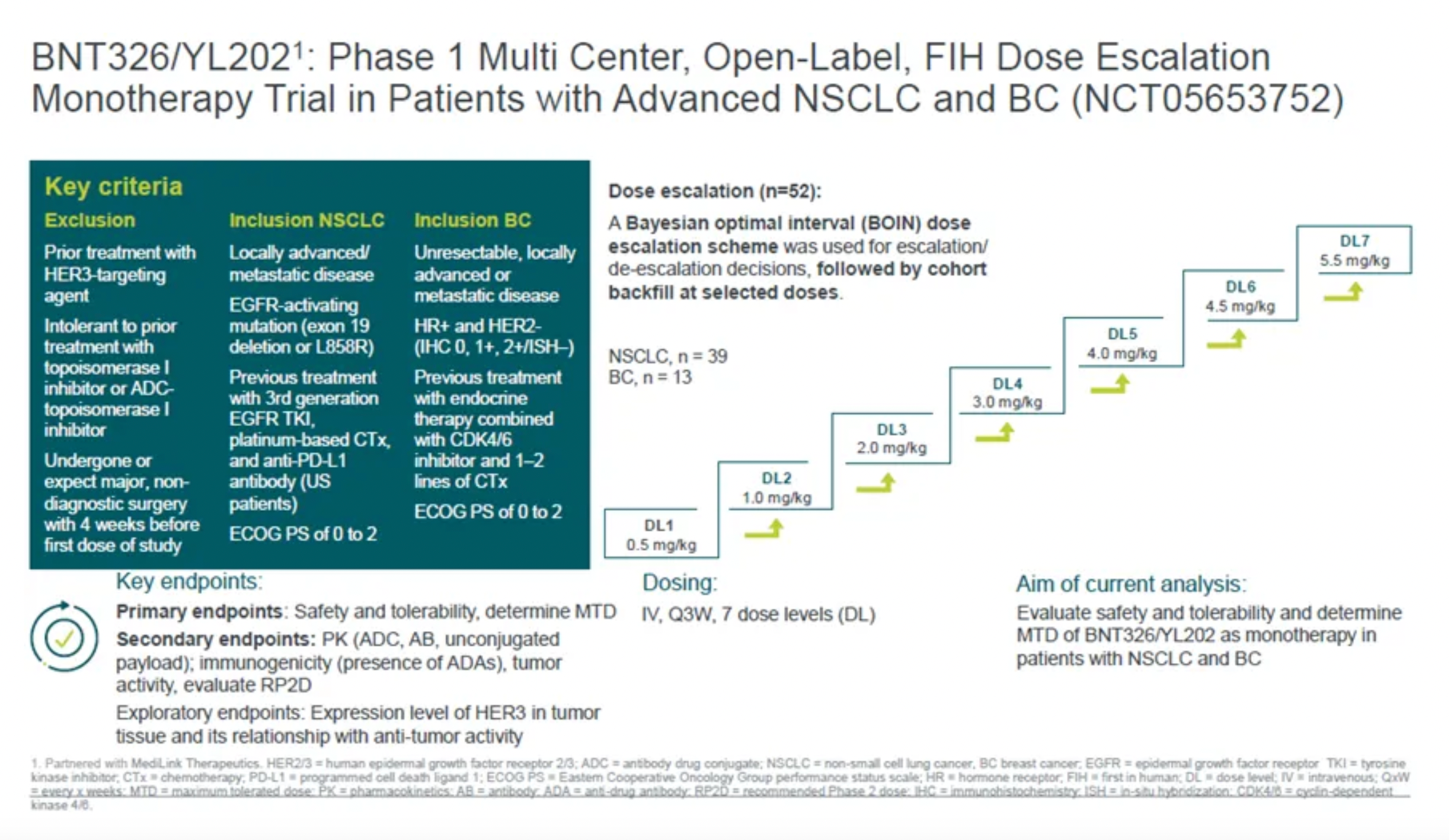
Three weeks earlier, BioNTech presented slides at the American Society of Clinical Oncology (ASCO) annual meeting, indicating that as of February 4, 54 patients had been treated with BNT326/YL202 in the Phase I trial. There were two patient deaths in the 4mpk (milligrams per kilogram) dosage group and one patient death in the 5.5mpk dosage group. Furthermore, in a report on June 4, BioNTech disclosed that a total of 14 patients had died during the treatment period, with 3 of those deaths attributed to drug-related side effects. For comparison, the recommended dose for DS8201 is 5.4mpk, administered via intravenous infusion every three weeks. The safety of this ADC from Medilink Therapeutics is undoubtedly in crisis.
What is the structure of this ADC, and why is it exhibiting such high toxicity?
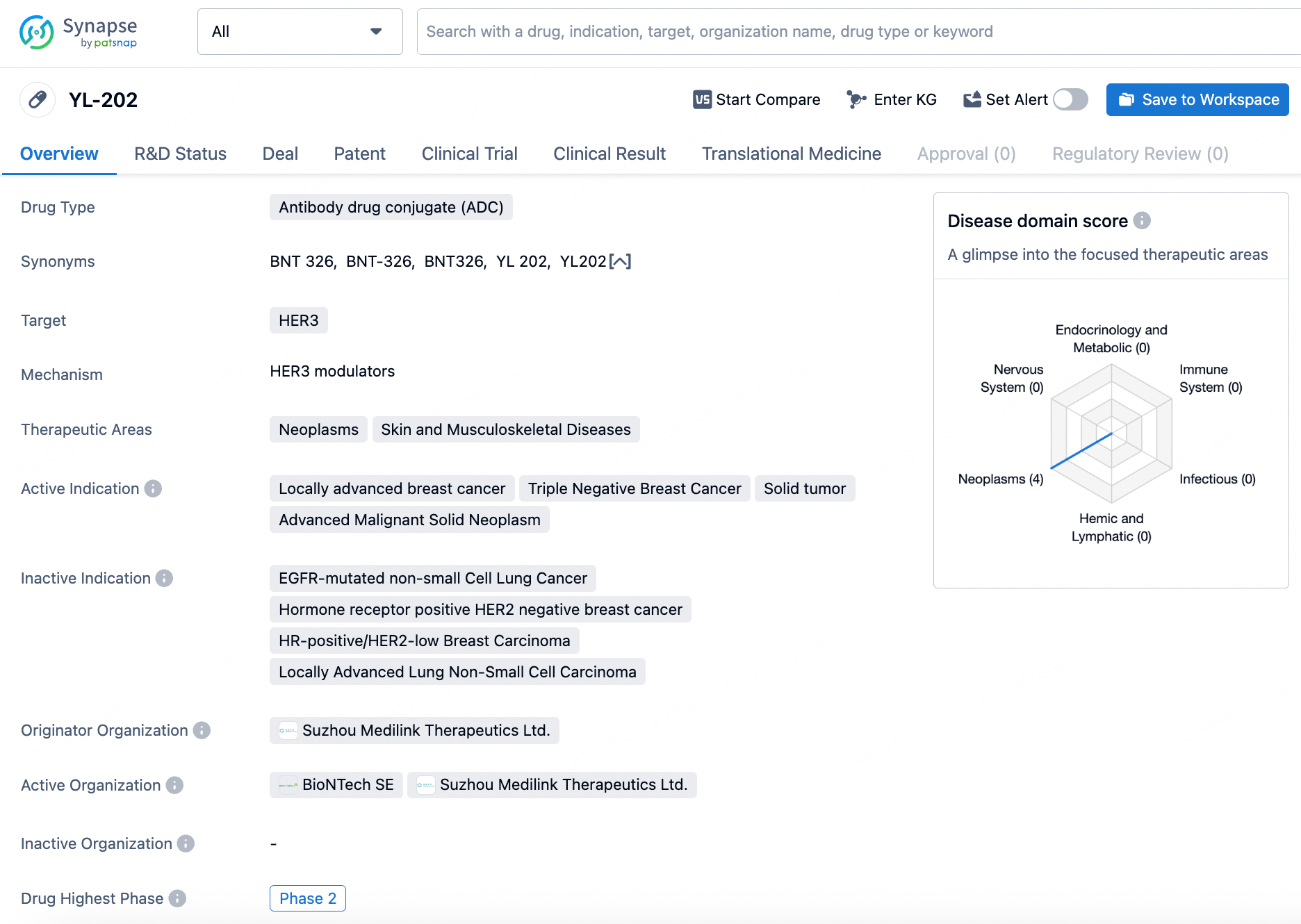
Entering the keywords BNT326/YL202 in the Synapse database yields 71 result entries, including information on drugs, drug transactions, news, clinical trials, clinical outcomes, and patents. Opening the patent section reveals three patents; among them, patent WO2023143263A1 protects YL-202. The earliest priority date is January 25, 2022, with the application date on January 17, 2023. The inventor is Chia-Chiang Tsai, one of the founders of Medilink Therapeutics.
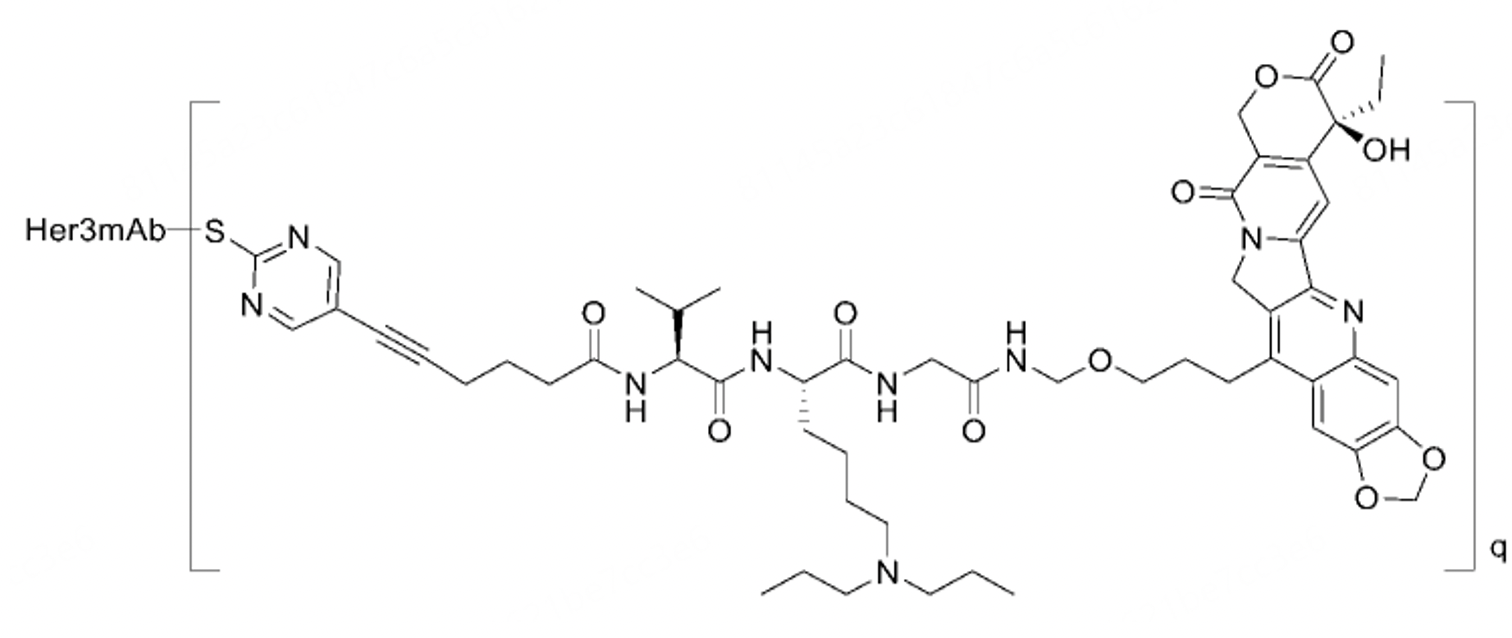
The structure of BNT326/YL202 is not disclosed in the public domain; it can only be inferred from this patent. According to the description in the patent, compared to the IgG1 group, doses of Her3-ADC-07 (with DAR8) at 5mg/kg and 1mg/kg significantly inhibit tumor growth in the NCI-H358 non-small cell lung carcinoma xenograft model, displaying a dose-dependent trend. The 1mg/kg group of Her3-ADC-07 demonstrates a TGI (Tumor Growth Inhibition) of 76.26%, slightly superior to the 5mg/kg group of Her3-ADC-21, which exhibits a TGI of 75.74%. The 1mg/kg group of Her3-ADC-21 has a TGI of 29.92%, somewhat stronger than the 1mg/kg Her3-C3-ADC-21 group's TGI of 24.11%. In patent table 10, the anti-Her3 antibody-drug conjugates tested for efficacy against SW480 colorectal carcinoma xenografts show significant inhibition of tumor growth at doses of 10mg/kg and 3mg/kg in the SW480 xenograft model, also demonstrating a dose-dependent trend.
Her3-ADC-07 at doses of 10mg/kg and 3mg/kg exhibited significant inhibitory effects on tumor growth in a SW480 human colorectal cancer xenograft model, and displayed a dose-dependent trend. Additionally, Her3-ADC-07 (DAR8) demonstrated good stability in the plasma of mice, rats, dogs, monkeys, and humans, with less than 1% of the biologically active molecule A1 released compared to the theoretical maximum concentration after 504 hours (21 days) of in vitro incubation.
Interestingly, in comparison to Daiichi Sankyo's Patritumab Deruxtecan, which is in phase three clinical trials, preclinical animal safety of Her3-ADC-07 was evaluated. In GLP toxicity trials, the highest non-severe toxic dose for Her3-ADC-07 was 10mpk, more than three times that of Patritumab Deruxtecan. However, this comparison was only made for the cynomolgus monkey species and solely based on literature data.
The structure of Her3-ADC-07 is depicted below. The primary difference from the comparator positive structure lies in the use of a linker from the Medilink Therapeutics platform. Although the toxin also has a camptothecin-like structure, it differs from the toxin Dxd used in Patritumab Deruxtecan and DS8201, replacing methyl and F groups with an oxygen-containing heterocycle in Dxd. Whether the minor alterations in the toxin or the instability of the platform's linker pose safety issues remains to be determined by further experimental results. If the issue originates from the platform linker, this could potentially cast a shadow on other molecules in the Medilink Therapeutics platform portfolio.
Based on current clinical observations of safety risks, Medilink Therapeutics plans to reduce the dosage in future development of YL202, focusing on doses below 4.0 mg per kilogram (mpk). However, even at the dose level of DL4 (3.0 mg/kg), while no deaths occurred, the incidence rate of Grade 3 or higher treatment-related adverse events (TRAEs) still reached 90.0%. The potential to achieve the expected efficacy and safety at doses below 4.0 mg/kg remains highly uncertain.
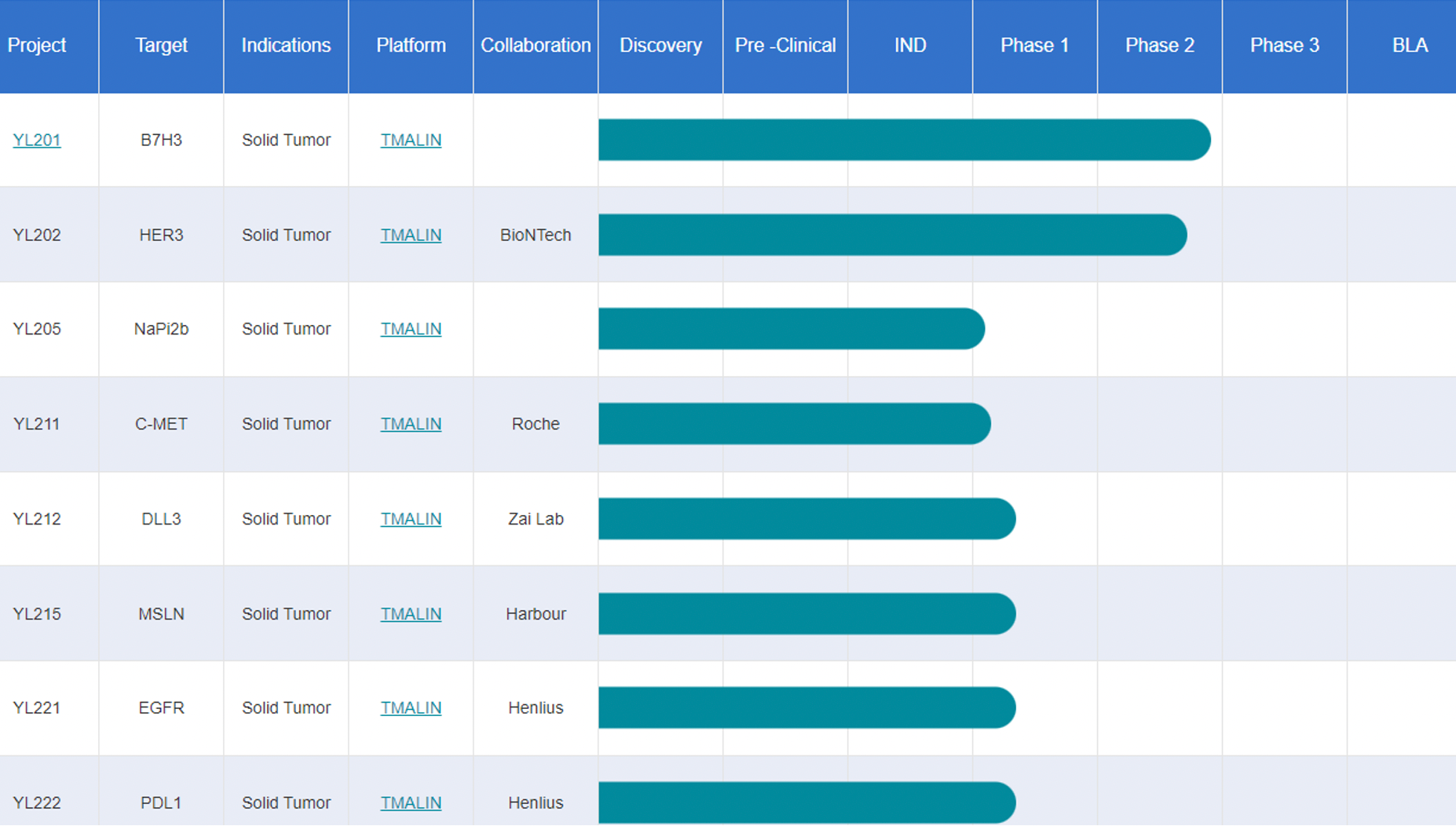
Medilink Therapeutics has developed several antibody-drug conjugate (ADC) pipelines based on its TMALIN platform, including YL201 targeting B7H3 in Phase II, YL202 targeting HER3 in Phase II, YL205 targeting NaPi2b in Phase I/II, YL211 targeting cMET in Phase I, YL212 targeting DLL3 in Phase I, YL215/ HBM9033 targeting MSLN, YL221/HLX42 targeting EGFR, and YL222/HLX43 targeting PD-L1. These projects have publicly disclosed an initial payment of approximately $200 million, with milestone payments reaching at least $5 billion and royalties on sales.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!