The European Union has approved Dupixent® (dupilumab) as the first targeted therapy for COPD patients
Regeneron Pharmaceuticals, Inc. and Sanofi announced that the European Commission has granted approval for Dupixent (dupilumab) as an additional maintenance therapy for adults with uncontrolled chronic obstructive pulmonary disease with elevated blood eosinophils. This approval is specified for patients who are already receiving a combination therapy that includes an inhaled corticosteroid, a long-acting beta2-agonist, and a long-acting muscarinic antagonist, or a combination of a LABA and a LAMA when ICS is unsuitable.
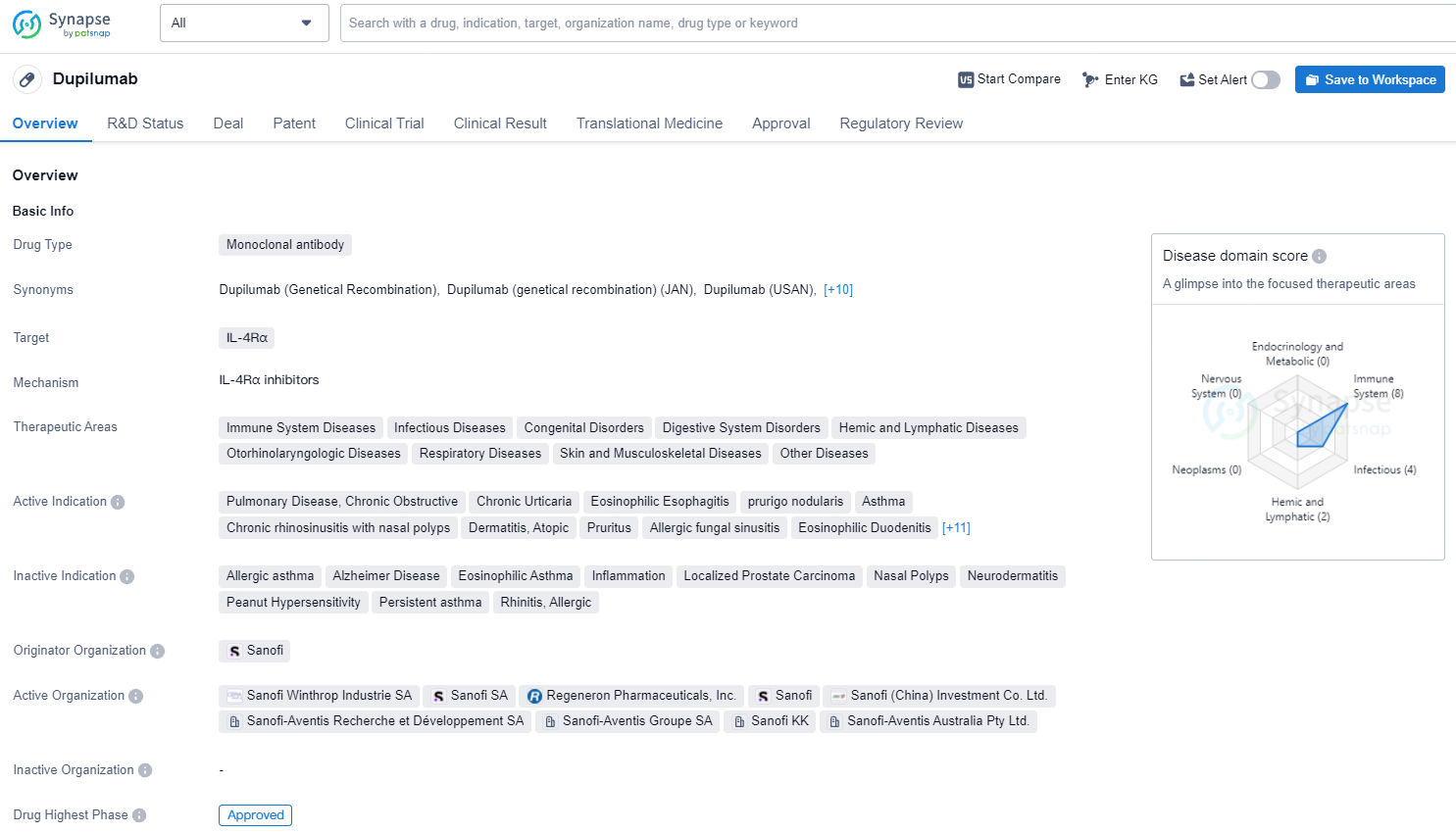
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The EC has made history by being the first regulatory body globally to greenlight Dupixent for COPD treatment. Other regulatory submissions are being reviewed internationally, including in the U.S., China, and Japan.
“COPD is a severe and progressive condition that causes shortness of breath, restricting simple daily activities like climbing stairs or walking to the mailbox. Many patients feel marginalized and lonely due to the disease’s physical and emotional impact,” stated Tonya Winders, President and CEO of Global Allergy & Airways Patient Platform.
“The endorsement of Dupixent for COPD marks a significant and long-anticipated breakthrough for those struggling to breathe during even minor tasks and facing risks of hospitalization, irreversible health decline, and despair,” remarked George D. Yancopoulos, M.D., Ph.D., Board Co-Chair, President, and Chief Scientific Officer at Regeneron, and one of the key inventors of Dupixent.
Yancopoulos added, “With this approval, we are proud that Dupixent has the potential to revolutionize treatment in another disease, serving as a pioneer therapy demonstrating remarkable improvements in exacerbations and lung function, along with enhancements in health-related quality of life as evidenced in two extensive Phase 3 trials.”
Safety results from both trials aligned largely with the known safety profile of Dupixent in its current indications. The most frequent side effects across indications include injection site reactions, conjunctivitis, allergic conjunctivitis, arthralgia, oral herpes, and eosinophilia. Notable adverse events more common with Dupixent compared to placebo in the COPD trials included back pain, COVID-19, diarrhea, headache, and nasopharyngitis. Additional injection site adverse reactions reported in the COPD trials were bruising, induration, rash, and dermatitis.
“Patients suffering from uncontrolled COPD have awaited a novel treatment path for years. We are excited to introduce the first biologic targeting an underlying cause of this debilitating disease to reduce COPD exacerbations and enhance lung function,” said Paul Hudson, CEO of Sanofi. “With today’s Dupixent approval, we have the opportunity to reshape treatment for over 200,000 patients in the EU living with uncontrolled COPD and elevated blood eosinophils. We are eager to collaborate with other regulators globally to swiftly bring this innovative treatment to more patients worldwide.”
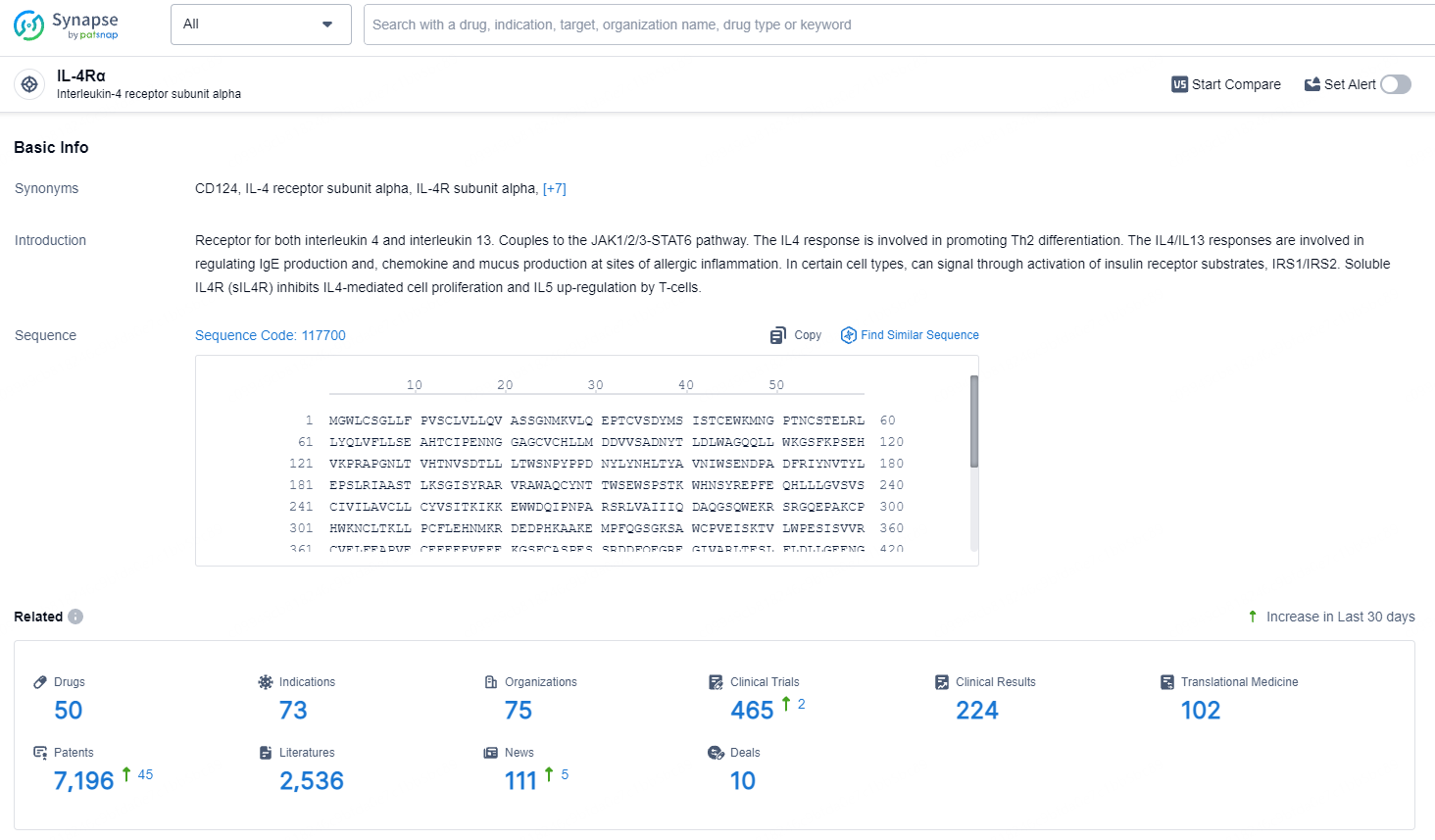
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of July 9, 2024, there are 50 investigational drugs for the IL-4Rα targets, including 73 indications, 75 R&D institutions involved, with related clinical trials reaching 465, and as many as 7196 patents.
Dupilumab is a monoclonal antibody drug that targets IL-4Rα and is used in the treatment of a wide range of therapeutic areas, including immune system diseases, infectious diseases, congenital disorders, digestive system disorders, hemic and lymphatic diseases, otorhinolaryngologic diseases, respiratory diseases, skin and musculoskeletal diseases, and other diseases. Dupilumab is a significant advancement in the field of biomedicine, offering a targeted treatment option for a wide range of diseases and conditions. Its approval in multiple markets and the various regulatory designations it has received highlight its potential to address unmet medical needs and improve patient outcomes. As an expert in the pharmaceutical industry, it is important to closely monitor the market performance and further developments related to Dupilumab, as well as potential collaborations and partnerships that could enhance its accessibility and impact.