Is Evinacumab approved by the FDA?
Evinacumab, marketed under the brand name Evkeeza, is a medication designed to manage a specific type of high cholesterol known as homozygous familial hypercholesterolemia (HoFH).Evinacumab was approved by the U.S. Food and Drug Administration (FDA) on February 11, 2021. This approval provides a crucial treatment option for patients, especially those for whom traditional cholesterol-lowering medications are not sufficient.
Uses and Administration
Uses:
- Evinacumab is used in conjunction with other cholesterol-lowering therapies to reduce low-density lipoprotein cholesterol (LDL-C) in adults and children aged 12 years and older with HoFH.
Administration:
- The medication is administered as an intravenous infusion, typically once a month.
- The infusion process takes about 60 minutes and must be conducted by a healthcare professional in a clinical setting.
Precautions and Considerations
Before Taking Evinacumab:
- Patients should inform their doctor of any known allergies to evinacumab.
- Evinacumab can harm an unborn baby; thus, women of childbearing age may need a pregnancy test before starting treatment and should use effective birth control during treatment and for at least five months after the last dose.
- It is advisable to discuss breastfeeding with a doctor while using this medication.
Potential Side Effects:
- Common Side Effects: Cold or flu symptoms (fever, chills, body aches), dizziness, pain in arms or legs, nausea, and lack of energy.
- Serious Side Effects: Allergic reactions like hives, rash, itching, feeling light-headed, wheezing, difficult breathing, and swelling of the face, lips, tongue, or throat.
- Some side effects may occur during the injection, such as dizziness, nausea, light-headedness, itching, sweating, headache, chest tightness, back pain, trouble breathing, or facial swelling. Medical caregivers are prepared to address these reactions promptly.
Patients are encouraged to report any side effects to their doctor or directly to the FDA at 1-800-FDA-1088.
Detailed Dosage Information
Usual Adult Dose for HoFH:
- 15 mg/kg via IV infusion once a month (every four weeks).
Usual Pediatric Dose for HoFH (12 years or older):
- 15 mg/kg via IV infusion once a month (every four weeks).
- LDL-C levels should be assessed periodically to monitor the effectiveness of the treatment, with measurements possible as soon as two weeks after initiating therapy.
Conclusion
Given its administration requirements and potential side effects, it is vital for patients to receive this treatment under the supervision of healthcare professionals. As with any medication, discussing personal medical history and potential risks with a healthcare provider is crucial for safe and effective treatment.
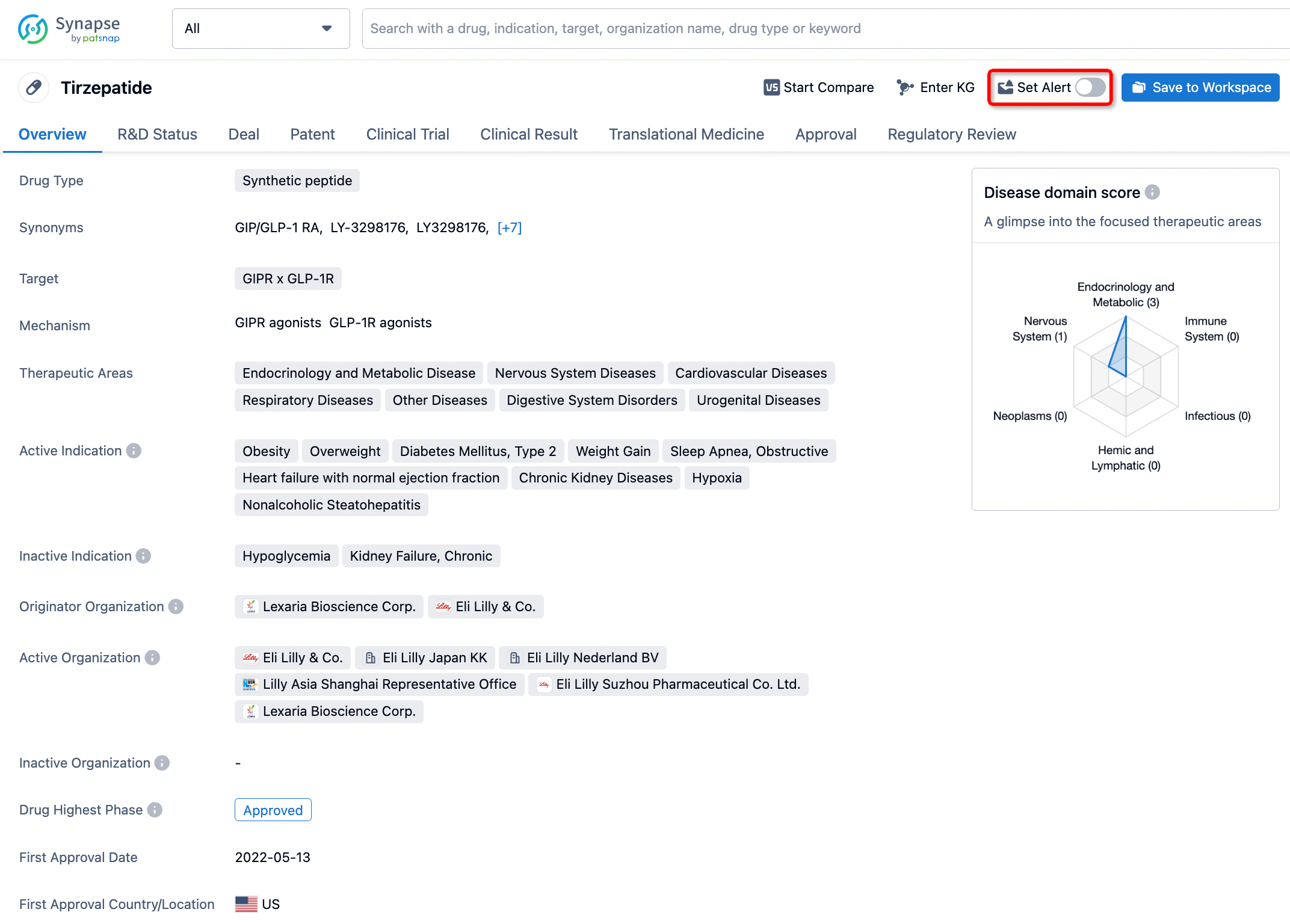
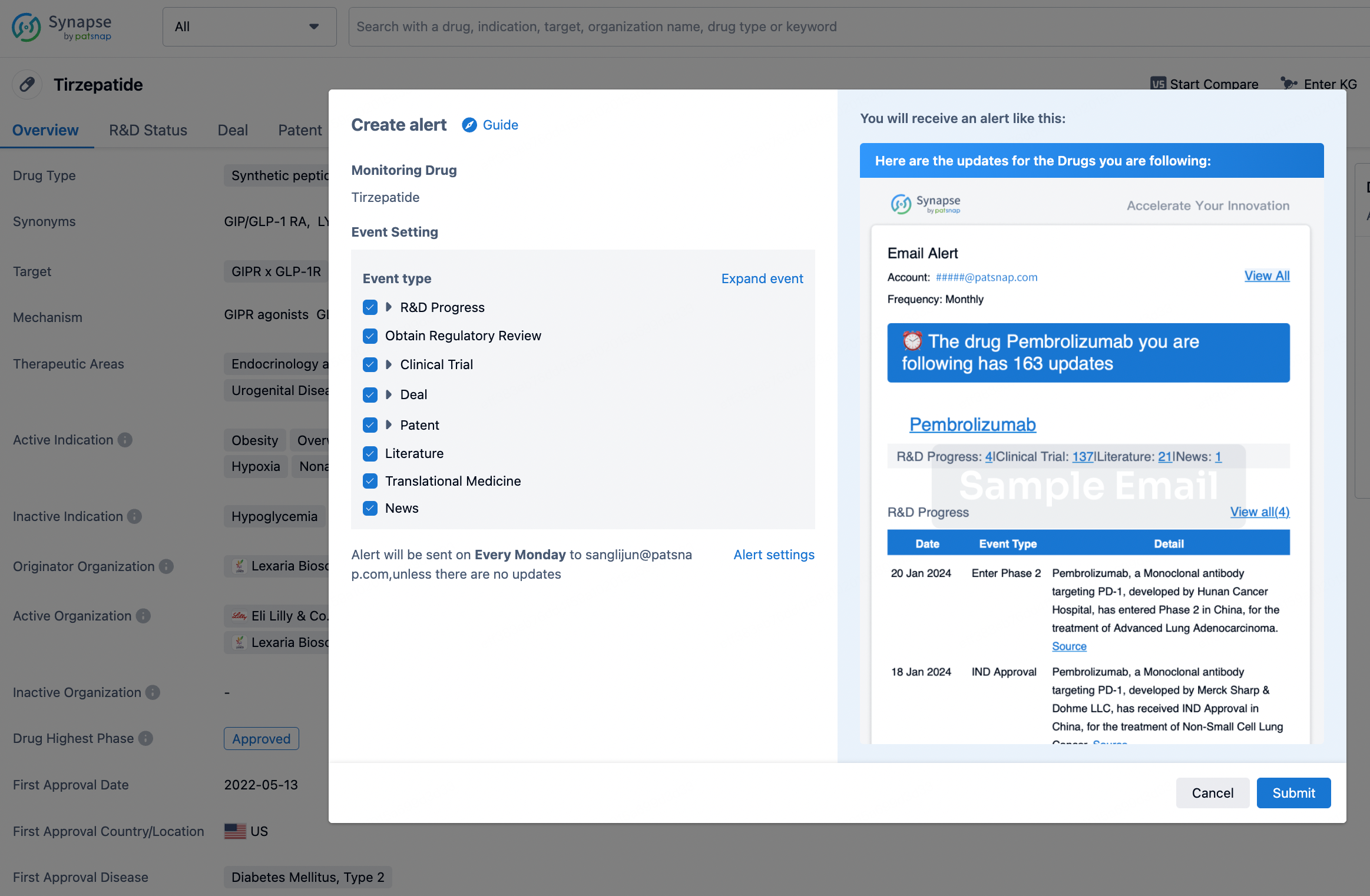
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!