The Evolving Competitive Landscape Behind Vyjuvek, the World’s First-ever Topical Gene Therapy
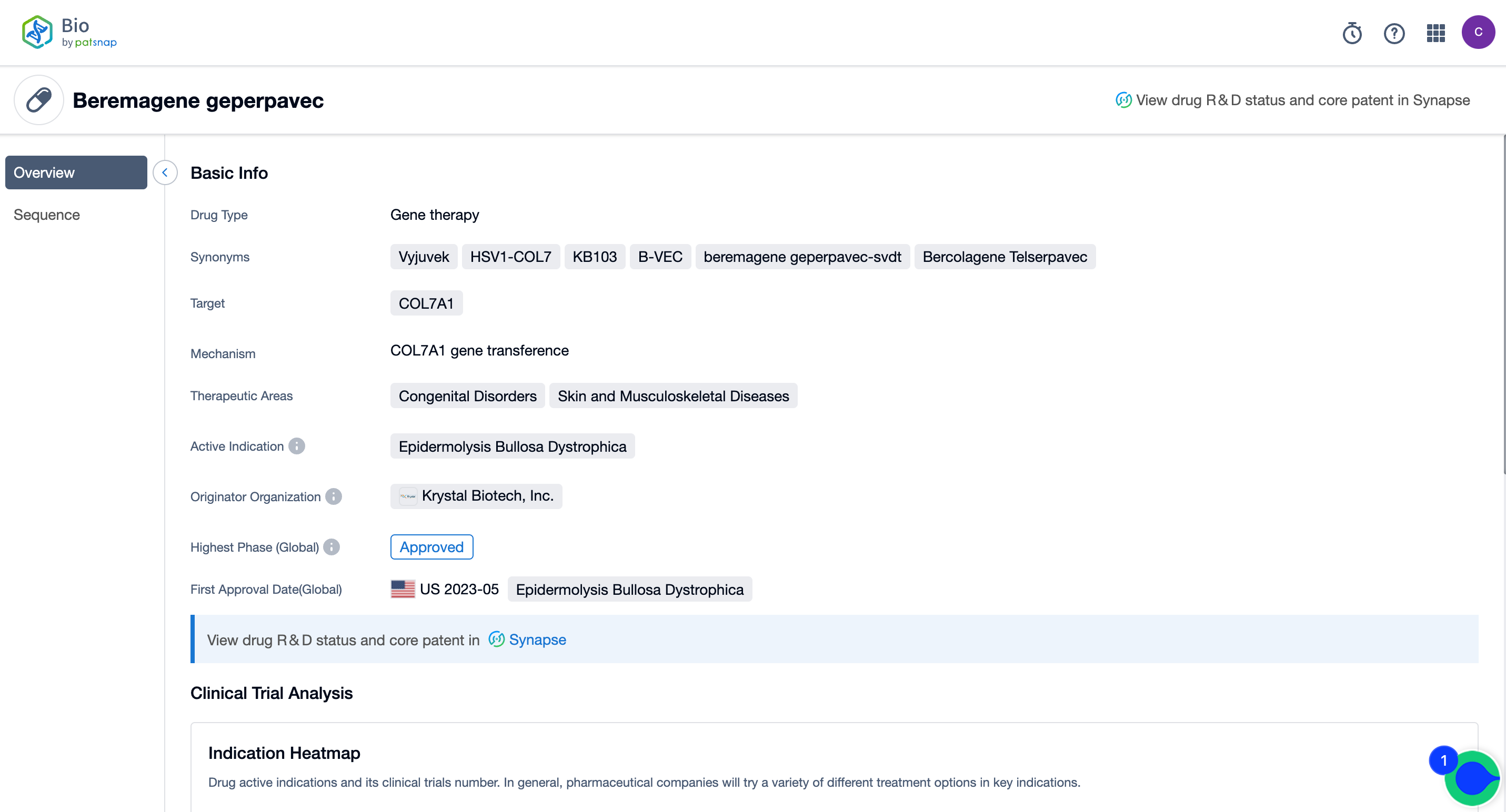
Krystal Biotech recently announced the company's third quarter earnings, attributing their positive return to the world's first topical gene therapy, Vyjuvek, which was approved by the FDA in May for the treatment of certain patients with Dystrophic Epidermolysis Bullosa (DEB). While Wall Street unanimously forecasted sales of Vyjuvek to reach $163 million next year, Evercore ISI raised their estimate to $273 million based on the company's release metrics provided on Monday.
Notably, after the drug received approval, the FDA issued a Priority Review Voucher (PRV) for Rare Pediatric Diseases to the company. This voucher grants priority review to subsequent drug applications that otherwise would not qualify for priority review. The PRV program is designed to encourage the development of new drugs for the prevention or treatment of rare diseases.
Vyjuvek Overview, Source, Patsnap Bio
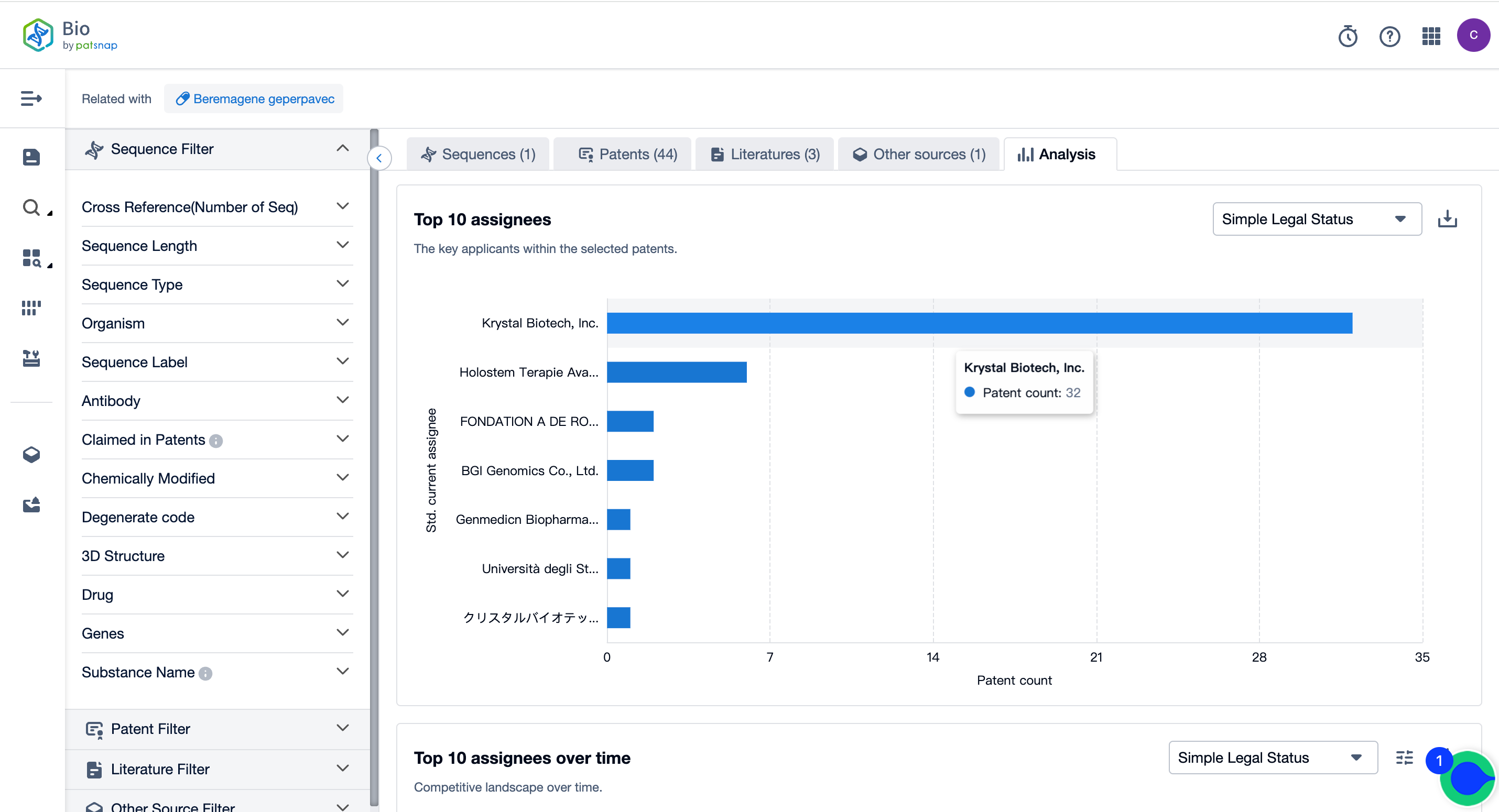
A Panoramic View of Vyjuvek’s Top 10 Patent Assignees
According to the Patsnap Bio Sequence Database, the top 10 applicants for the Vyjuvek patent are Krystal Biotech with 32 applications, Holostem Terapie Avanzate Srl with 6 applications, and FONDATION A DE ROTHSCHILD with 2 applications. For more applicants, please refer to the image below. Click on the bar chart to open it directly to view all the patent details for each assignee.
Source, Patsnap Bio
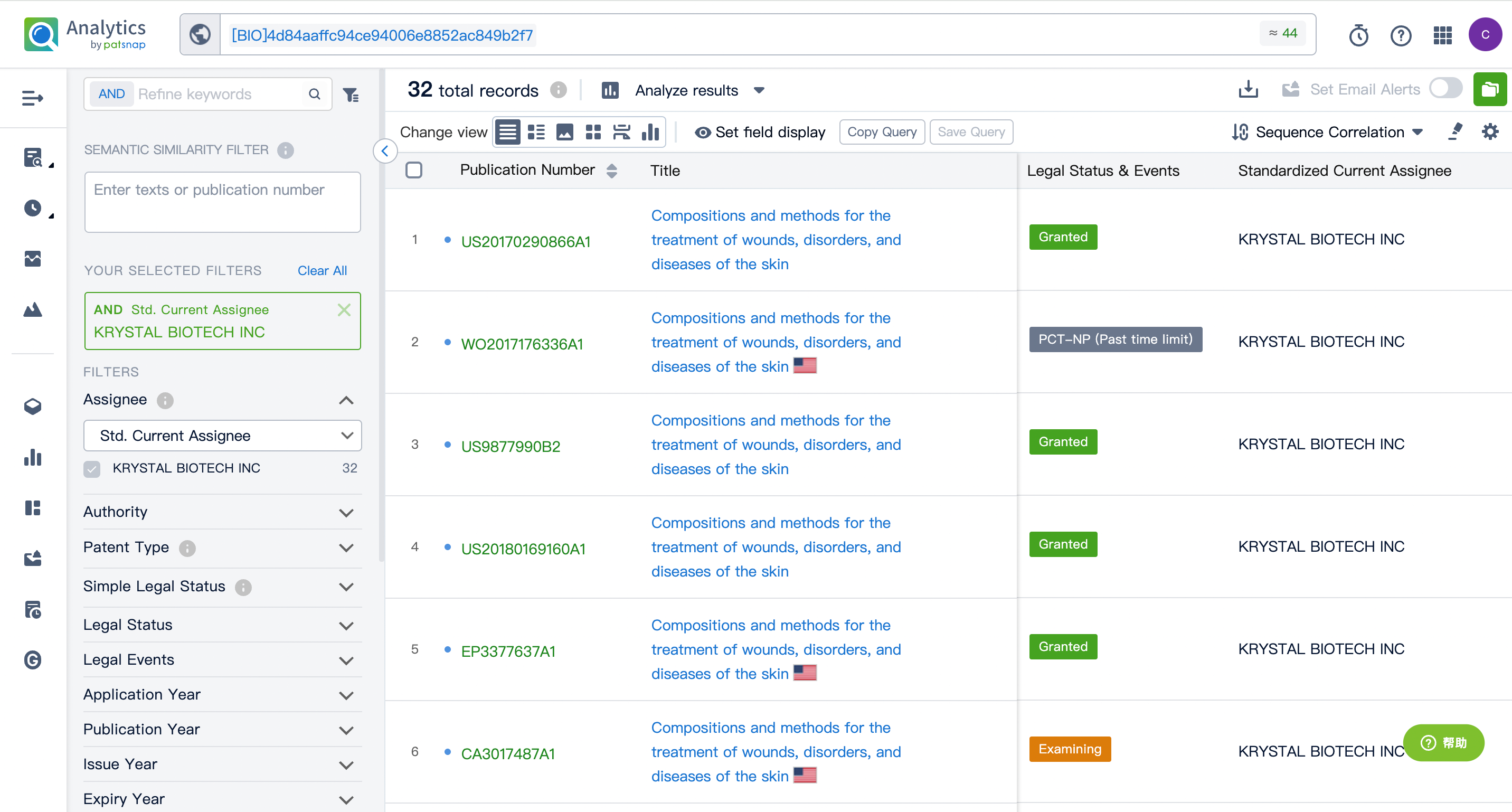
Evolution of the Layout of Top 10 Patent Assignees over the years, the Competitive landscape over time
Exploring the changes occurring within the application structure amongst Vyjuvek's top 10 patent assignees over time delivers insights into the competitive landscape's evolution.
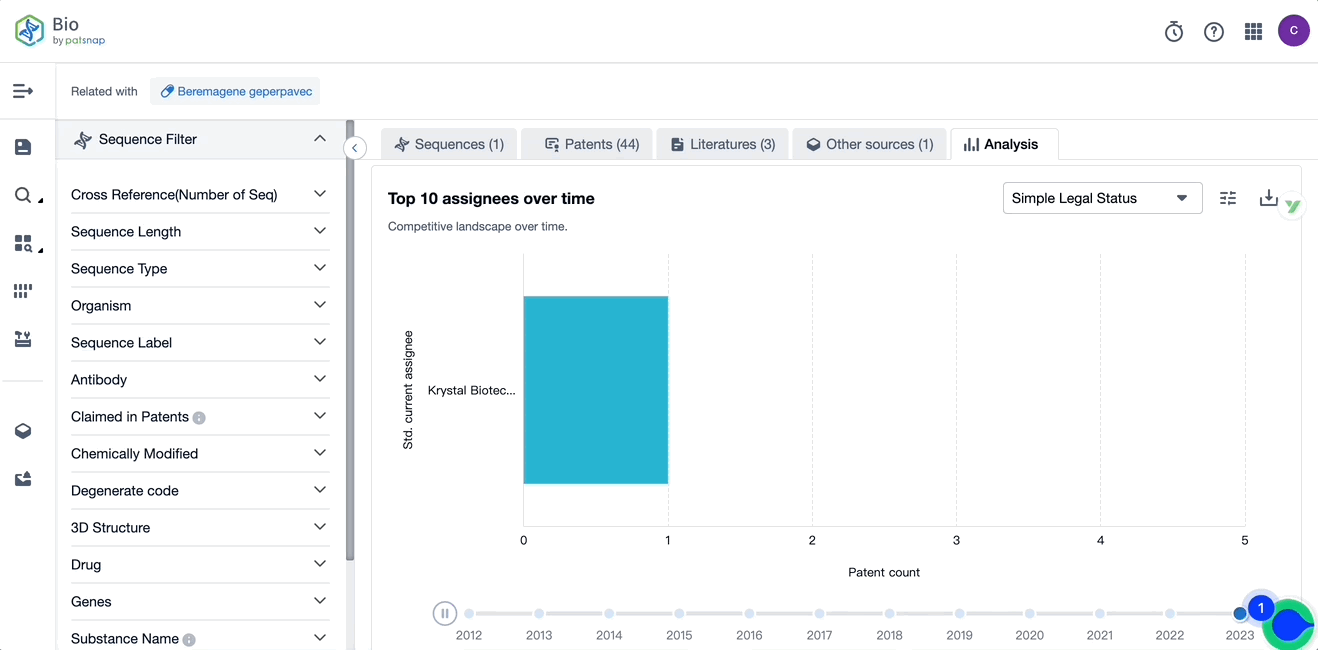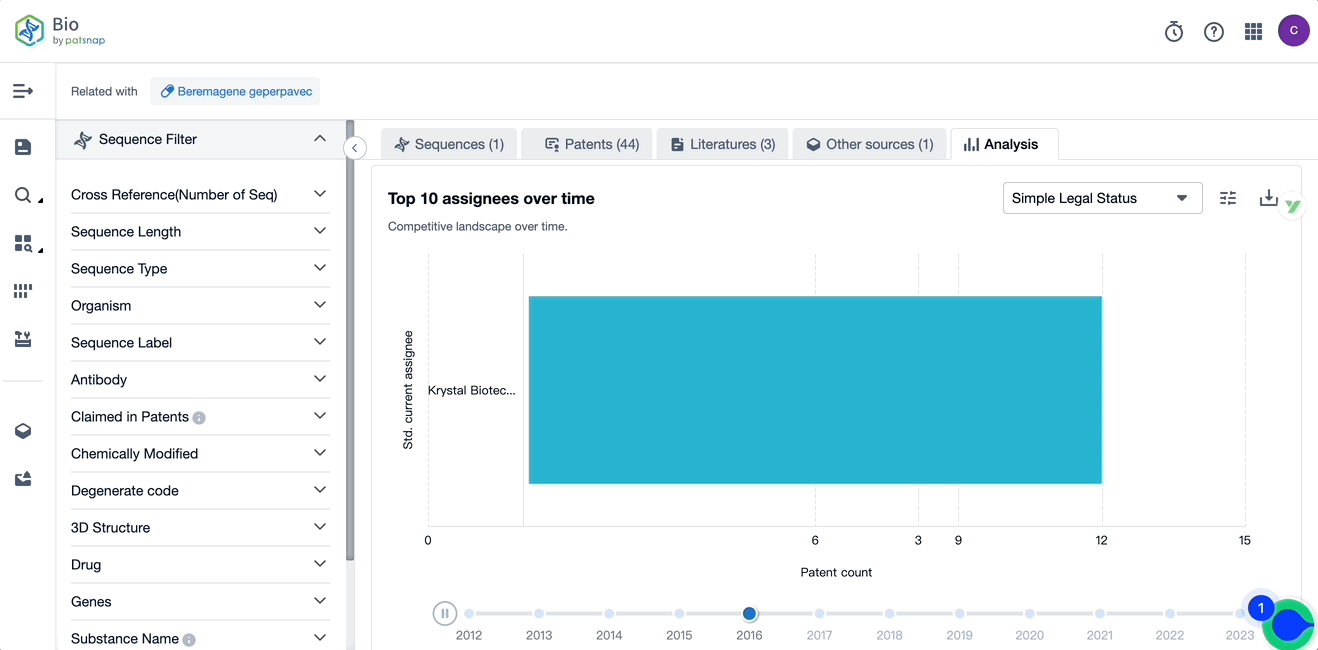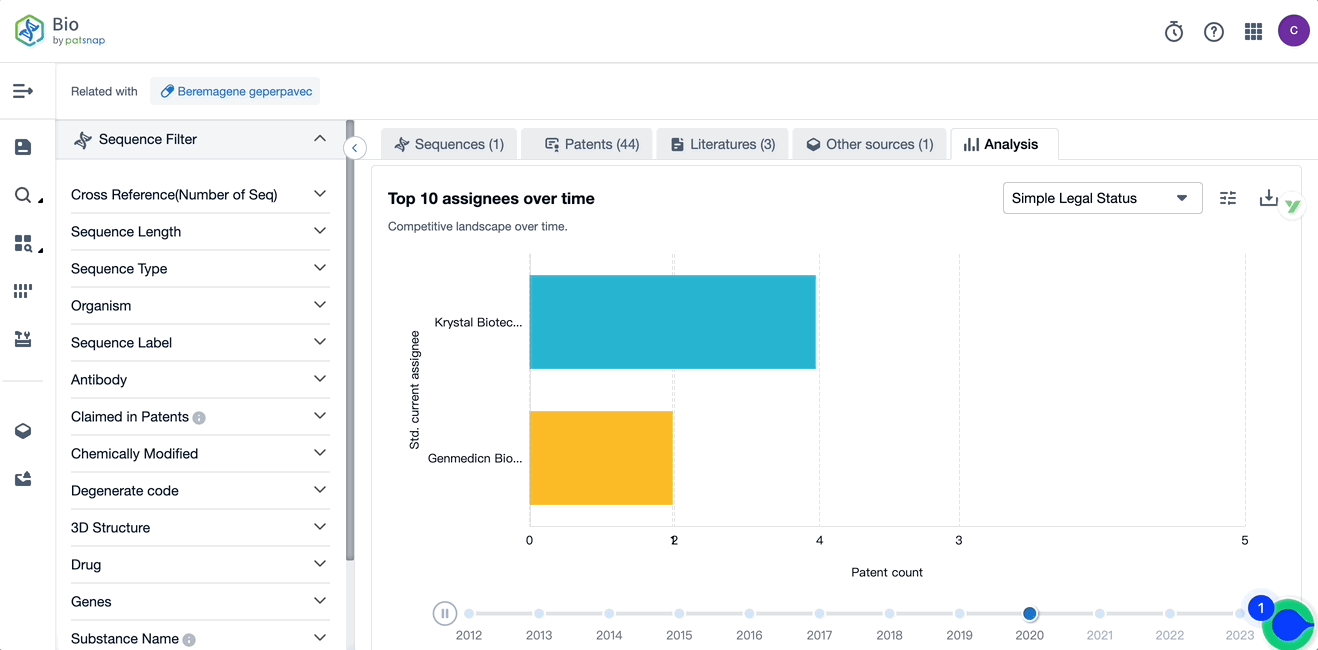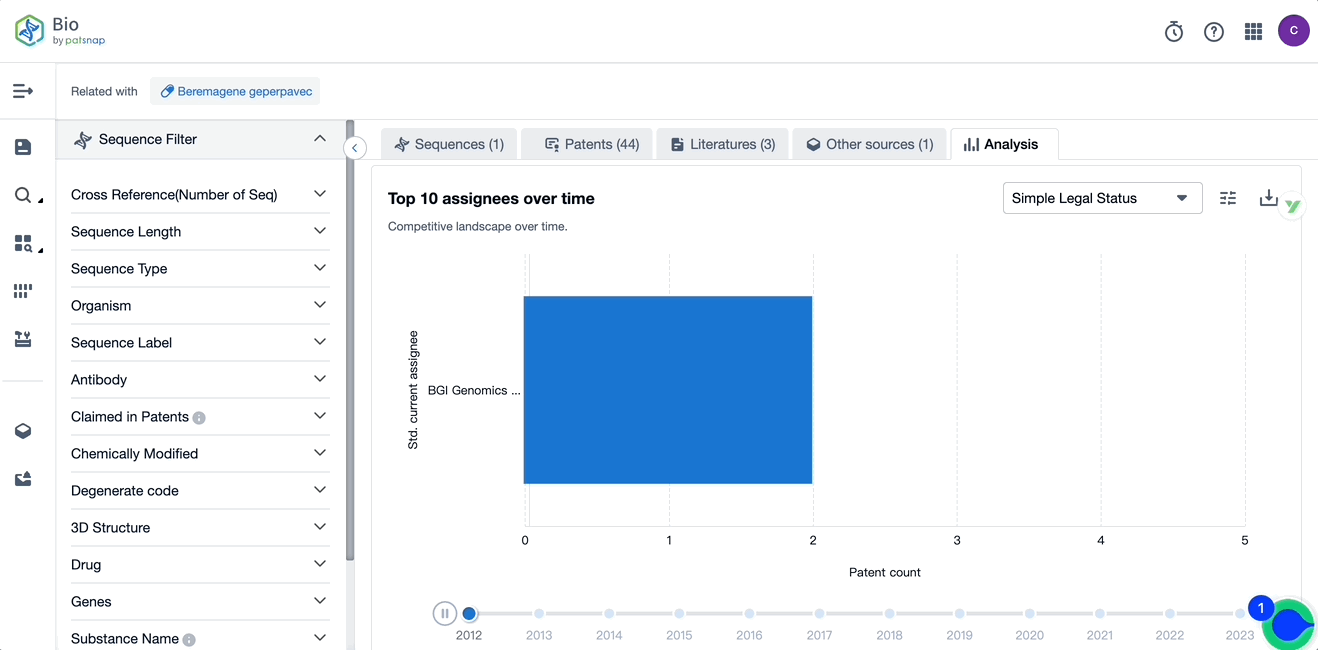
Source, Patsnap Bio
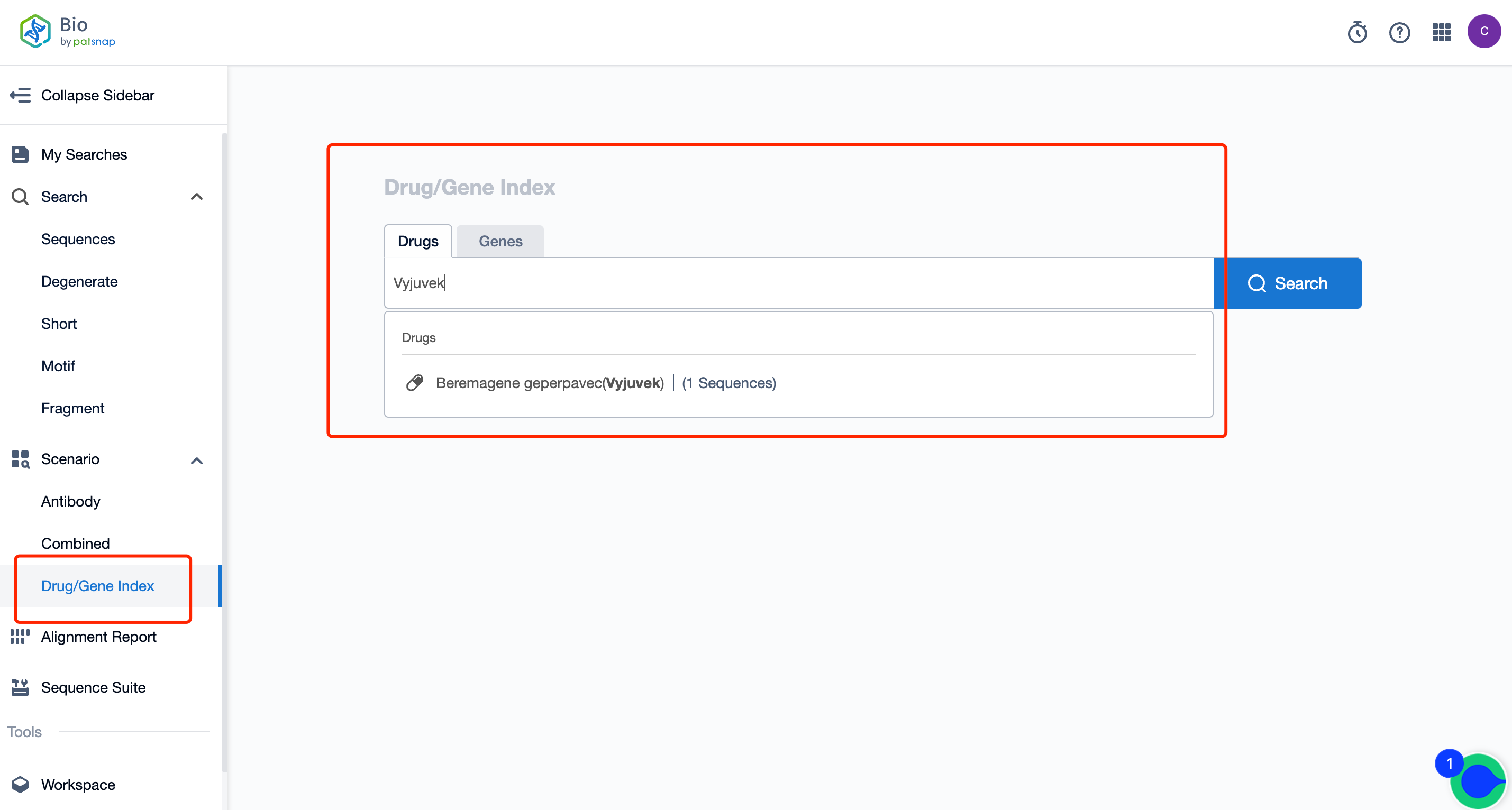
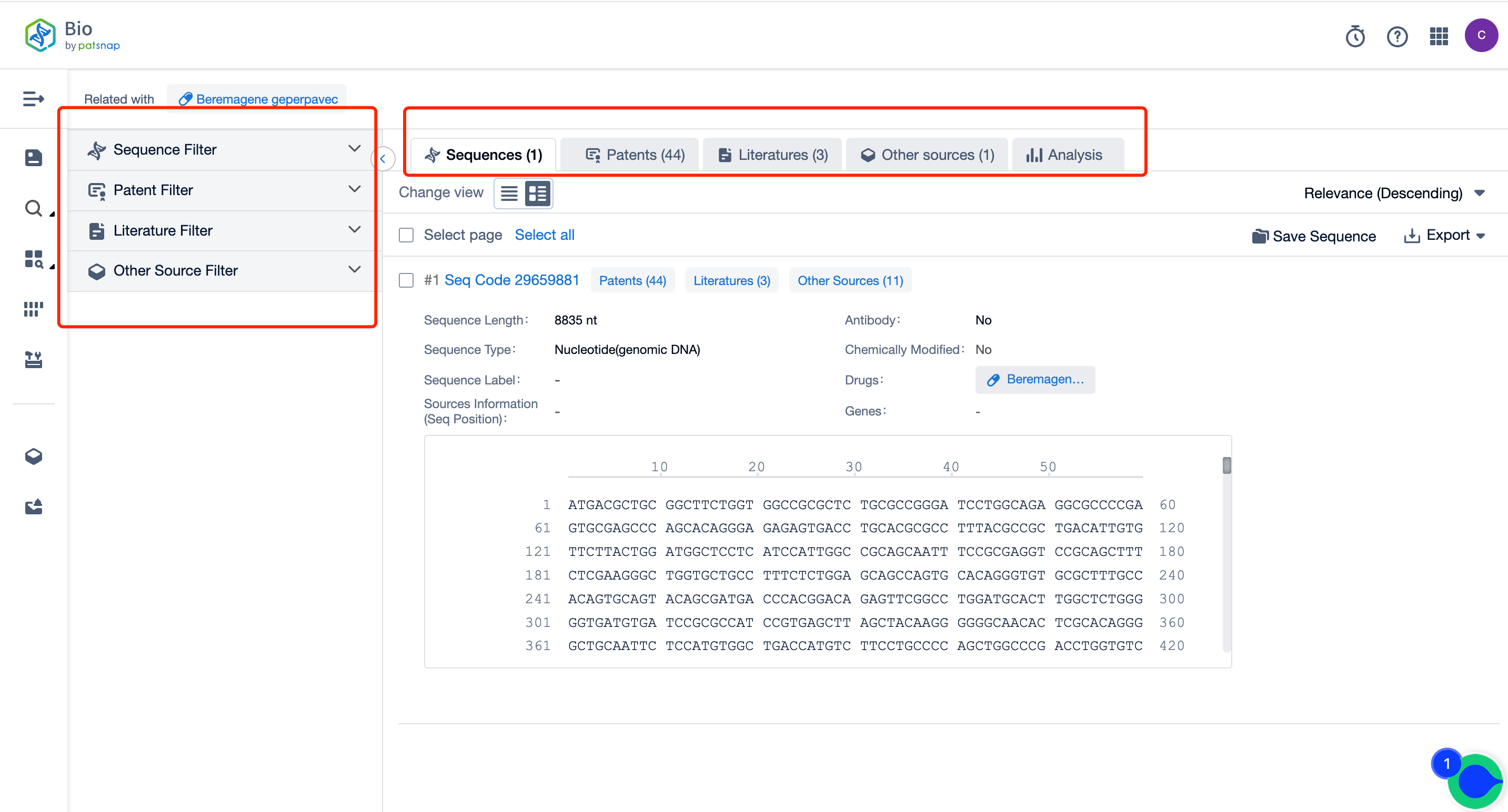
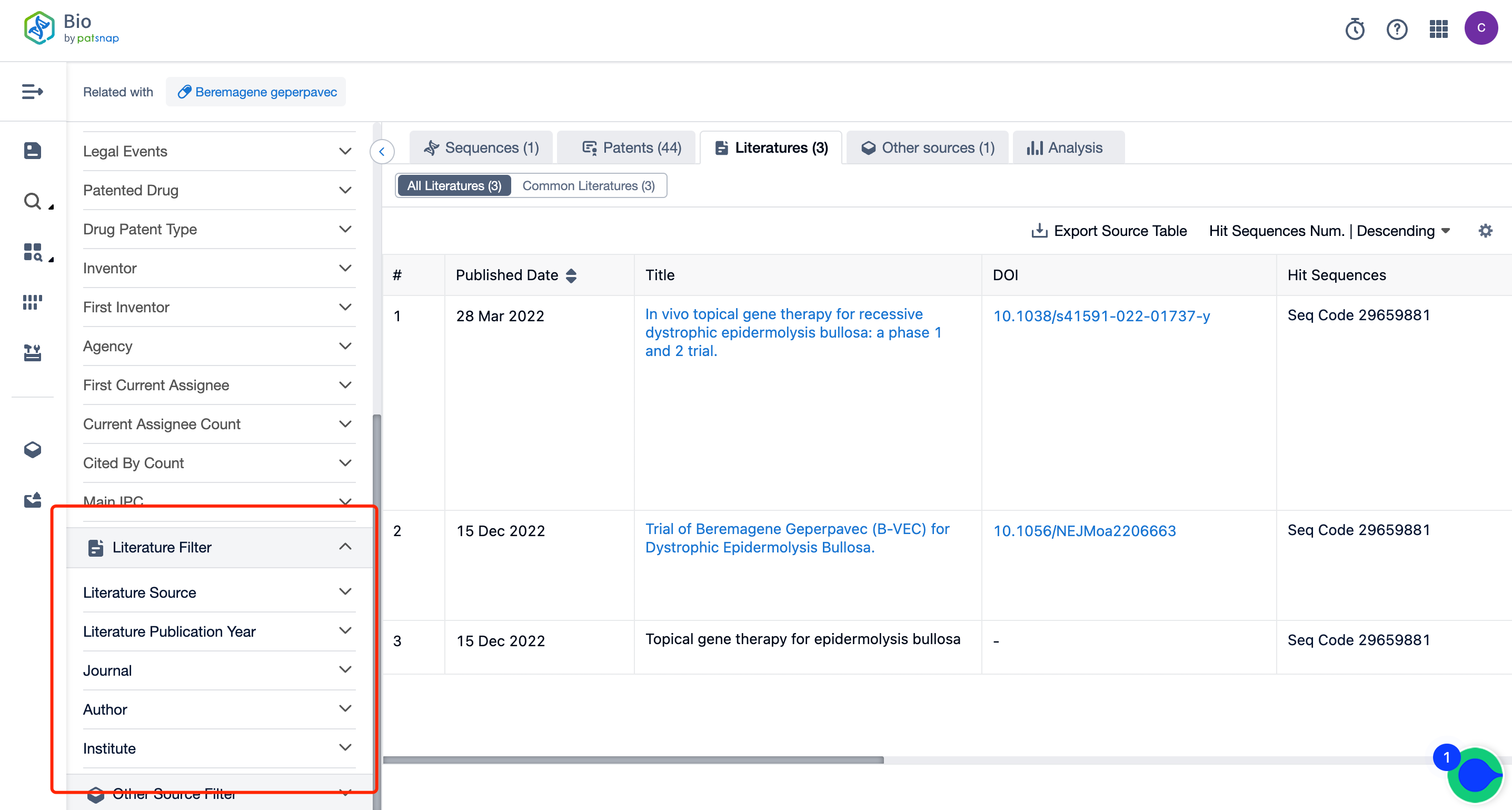
How Can You Acquire Comprehensive Data on Vyjuvek Sequence Patents for Free?
Firstly, establish a free account with Patsnap Bio Sequence Database. Proceed to the homepage's "standard search" and enter the Vyjuvek sequence or directly input the drug name, Vyjuvek, in the "Drug/Gene index.” This single action will unravel extensive details of Vyjuvek 's sequence, patent, literature, data from diversified sources, and a visually competitive landscape of patents.
The left panel's search result details page is equipped with an exhaustive range of filters, enabling you to pinpoint specific data accurately, thereby boosting your search experience and overall efficiency. Clicking on each data point will unfold a rich and detailed data set and a host of advantageous and practical tools to support your research process.
It is important to note that Patsnap Bio is the most extensive sequence search platform for the Patsnap database. It incorporates AI with human-curated data for comprehensive handling of protein and nucleotide sequence data plucked from global patents, biological periodicals, and public repositories. Essential biological sequences are manually annotated, illuminating structural modifications to provide the most accurate sequence data and boost sequence retrieval efficiency.
Free registration is available for the Bio biological sequence database: https://bio.patsnap.com. Act now to expedite your sequence search tasks.