ImmuneSensor Therapeutics Initiates Phase 1 Trial of IMSB301, a Leading cGAS Inhibitor
ImmuneSensor Therapeutics, a biotherapeutics firm currently in clinical development, is dedicated to creating first- and best-in-class cGAS-STING pathway inhibitors and agonists. These agents aim to target a variety of conditions related to inflammation, autoimmunity, and oncology across both peripheral and central nervous systems. The company announced that it has successfully administered the initial dose level cohort of healthy participants in a Phase 1 clinical trial, which is randomized, double-blind, and placebo-controlled. This trial focuses on their premier oral cGAS inhibitor candidate, IMSB301, designed for treating various inflammatory and autoimmune disorders. The foundation of the company’s technology stems from the research of Dr. Zhijian “James” Chen, whose innovative contributions earned him the prestigious 2024 Albert Lasker Basic Medical Research Award.
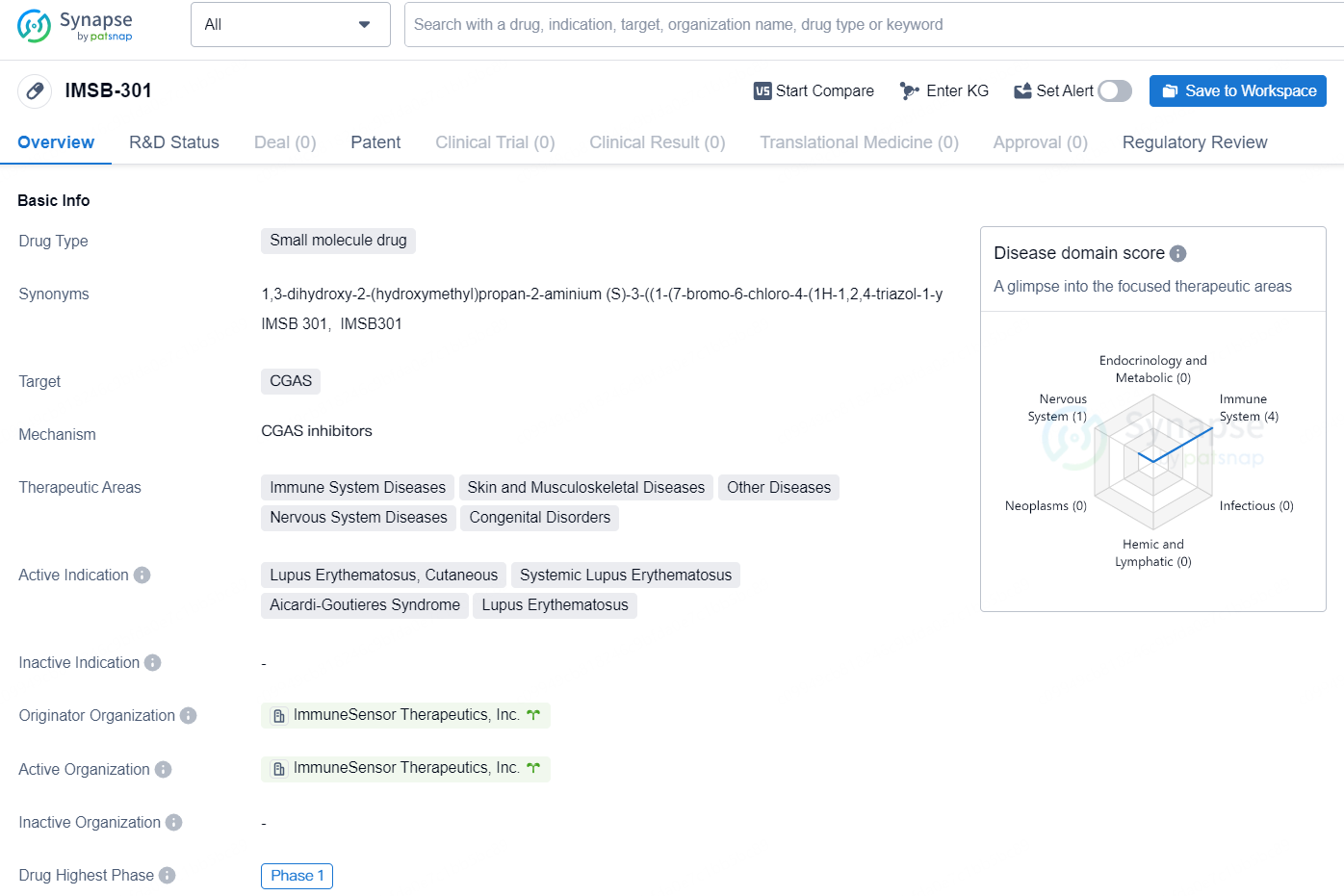
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"The cGAS-STING pathway plays a crucial role in mediating inflammation, and targeting the cGAS enzyme presents a promising therapeutic strategy for addressing various unmet medical challenges associated with inflammatory diseases," stated Tom Dubensky, Ph.D., president and CEO of ImmuneSensor. "We expect to obtain data regarding safety, pharmacokinetics, and target engagement from our Phase 1 trial involving healthy subjects by the end of 2024, which will facilitate a seamless transition to Phase 1b/2 clinical trials in patients with severe inflammatory disorders, particularly Type 1 interferonopathies. Our clinical protocol will initially focus on assessing IMSB301 in individuals diagnosed with Aicardi-Goutières syndrome (AGS), a rare inflammatory condition resulting from persistent cGAS pathway activation. Following this, we will evaluate IMSB301 in specific patient groups with cutaneous lupus erythematosus (CLE) and systemic lupus erythematosus (SLE)."
ImmuneSensor's Phase 1 clinical trial for IMSB301 is a randomized, placebo-controlled, double-blind study taking place in Australia with healthy volunteers. The main objectives of the trial include evaluating safety and tolerability, as well as pharmacokinetics (PK) and the degree of cGAS target engagement (inhibition). The study aims to recruit groups of eight participants each (comprising two receiving placebo and six receiving IMSB301 at each dosage), with plans for up to five single ascending dose (SAD) levels, followed by up to three multiple ascending dose (MAD) levels. Pharmacokinetics will be analyzed in both SAD and MAD segments, while cGAS target engagement will be assessed in the MAD segment using an ex vivo whole blood DNA stimulation assay. The initial dose group in the SAD segment has been completed, with exposure levels and pharmacokinetics aligning with predictions from nonclinical IND-enabling research.
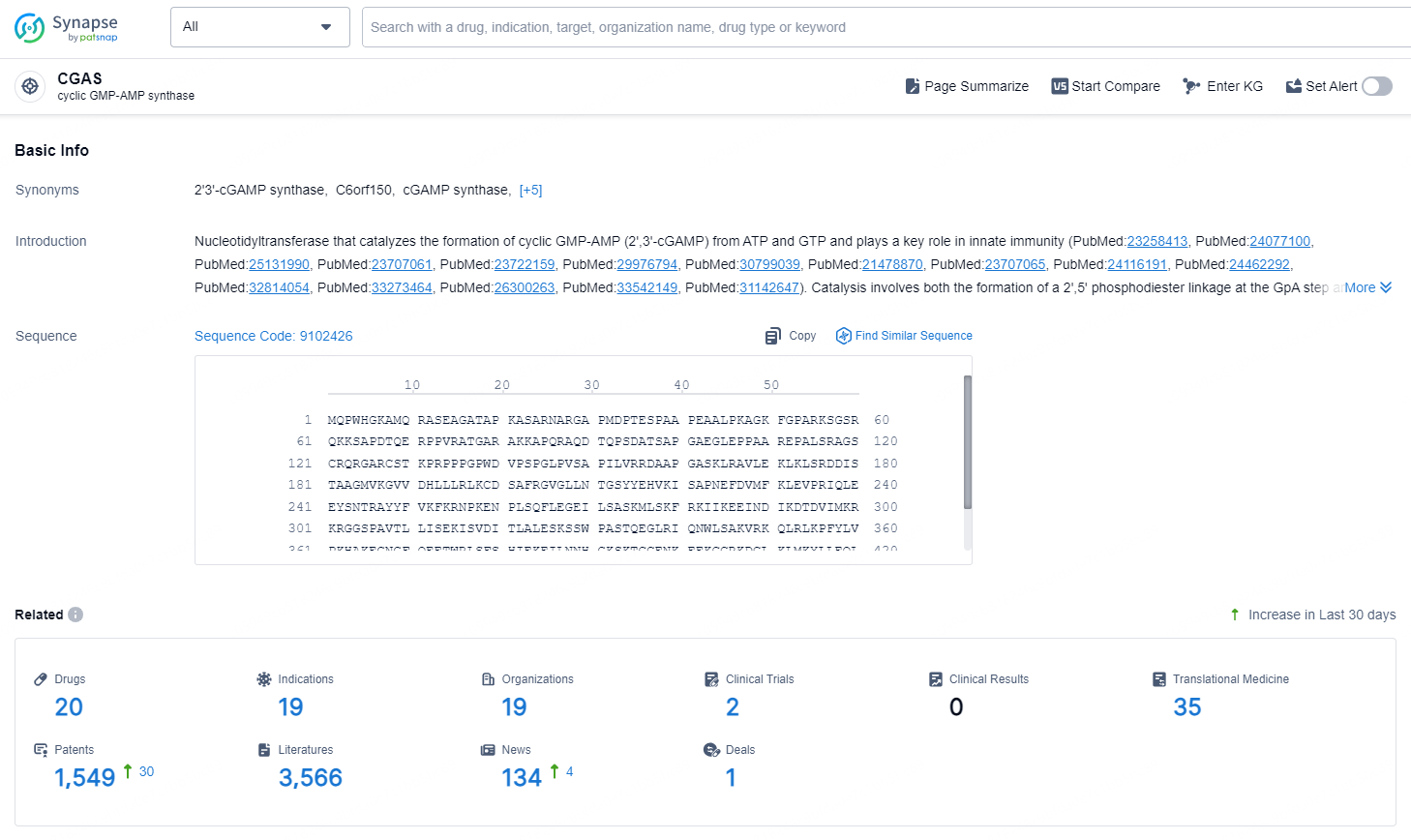
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of October 11, 2024, there are 20 investigational drugs for the CGAS targets, including 19 indications, 19 R&D institutions involved, with related clinical trials reaching 2, and as many as 1549 patents.
IMSB-301 is a small molecule drug developed by ImmuneSensor Therapeutics, Inc., with a focus on targeting CGAS. The therapeutic areas for this drug include immune system diseases, skin and musculoskeletal diseases, other diseases, nervous system diseases, and congenital disorders. The active indications for IMSB-301 are Lupus Erythematosus (both cutaneous and systemic) and Aicardi-Goutieres Syndrome.