The initial participant enrolled in a Phase 2 trial of Olatec's dapansutrile for Type 2 diabetes patients
Olatec Therapeutics, Inc., a pioneer in the field of selective NLRP3 inhibitors, revealed that individuals with type 2 diabetes mellitus and associated diabetic complications are currently being recruited for a Phase 2 clinical study taking place in Basel, Switzerland.
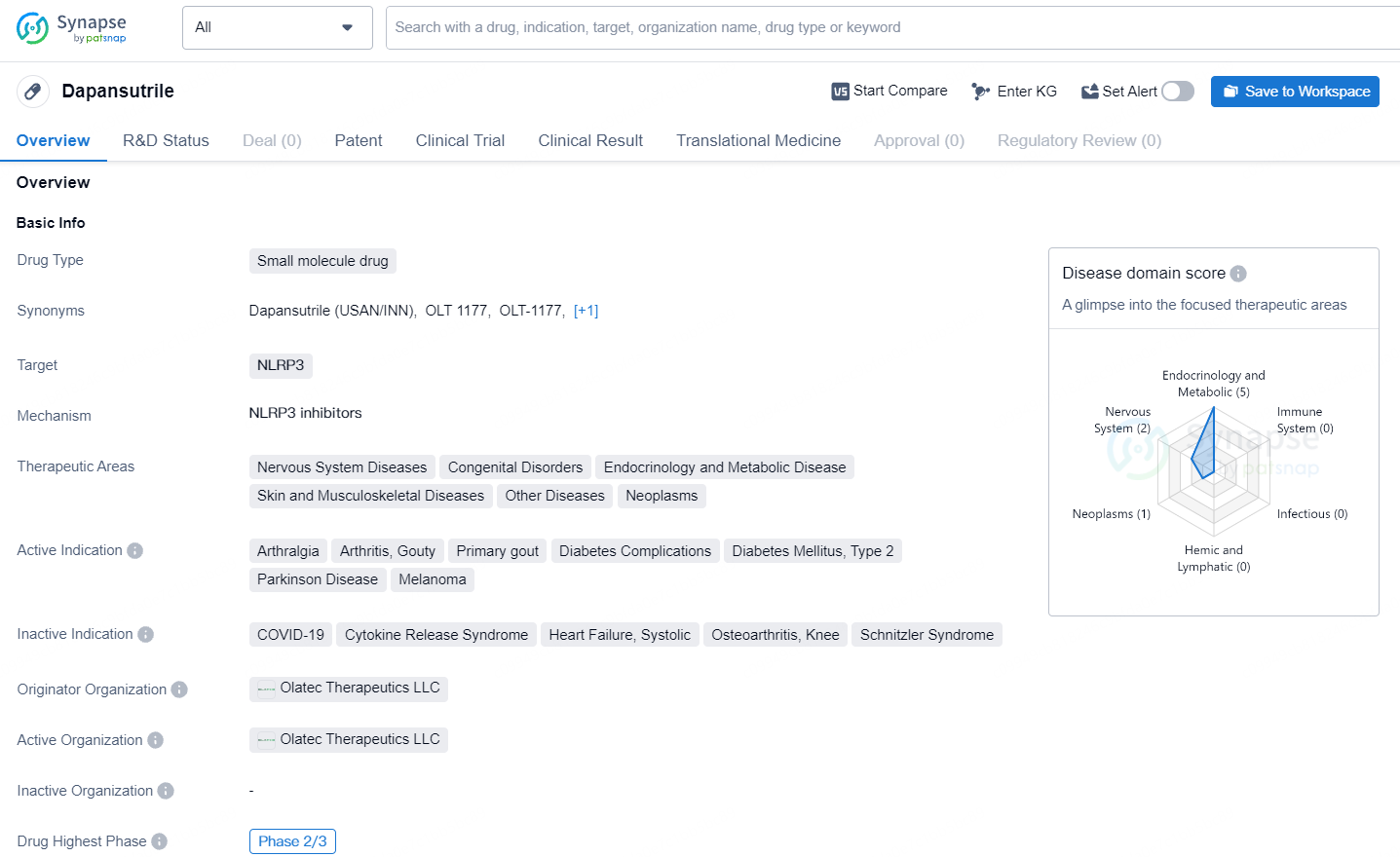
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The DAPAN-DIA Study is a multi-center, randomized, double-blind, placebo-controlled trial assessing the efficacy and safety of dapansutrile in individuals with T2D and complications related to diabetes. The study aims to enroll about 300 patients who, at the onset of the study, exhibit low-grade inflammation, obesity, and poorly managed glycemia despite standard anti-diabetic treatment. Participants will receive either dapansutrile or a placebo for six months.
"We believe that the data from this trial, including the combination with GLP-1 therapy, will be crucial for understanding the full potential of anti-inflammatory therapy using an NLRP3 inhibitor in this context," stated Mustafa Noor MD, Chief Medical Officer at Olatec Therapeutics.
The research is being conducted as an investigator-sponsored study under Principal Investigator Marc Donath MD at the University Hospital of Basel in Switzerland, a long-time collaborator and advisor to Olatec and a leading expert in immuno-metabolism.
Dr. Donath remarked, "There is a significant unmet need for effective T2D treatments that go beyond glycemic control to address the underlying inflammatory aspect of the disease and its cardiometabolic complications. The innovative DAPAN-DIA Study could allow dapansutrile to make a significant advance in T2D management."
Damaris Skouras, Founder and CEO of Olatec, commented: "Building on our previous research in heart failure and gout, the DAPAN-DIA Study marks a significant milestone in the development of dapansutrile for inter-related cardiometabolic diseases linked by chronic low-grade inflammation due to NLRP3/IL-1 activation."
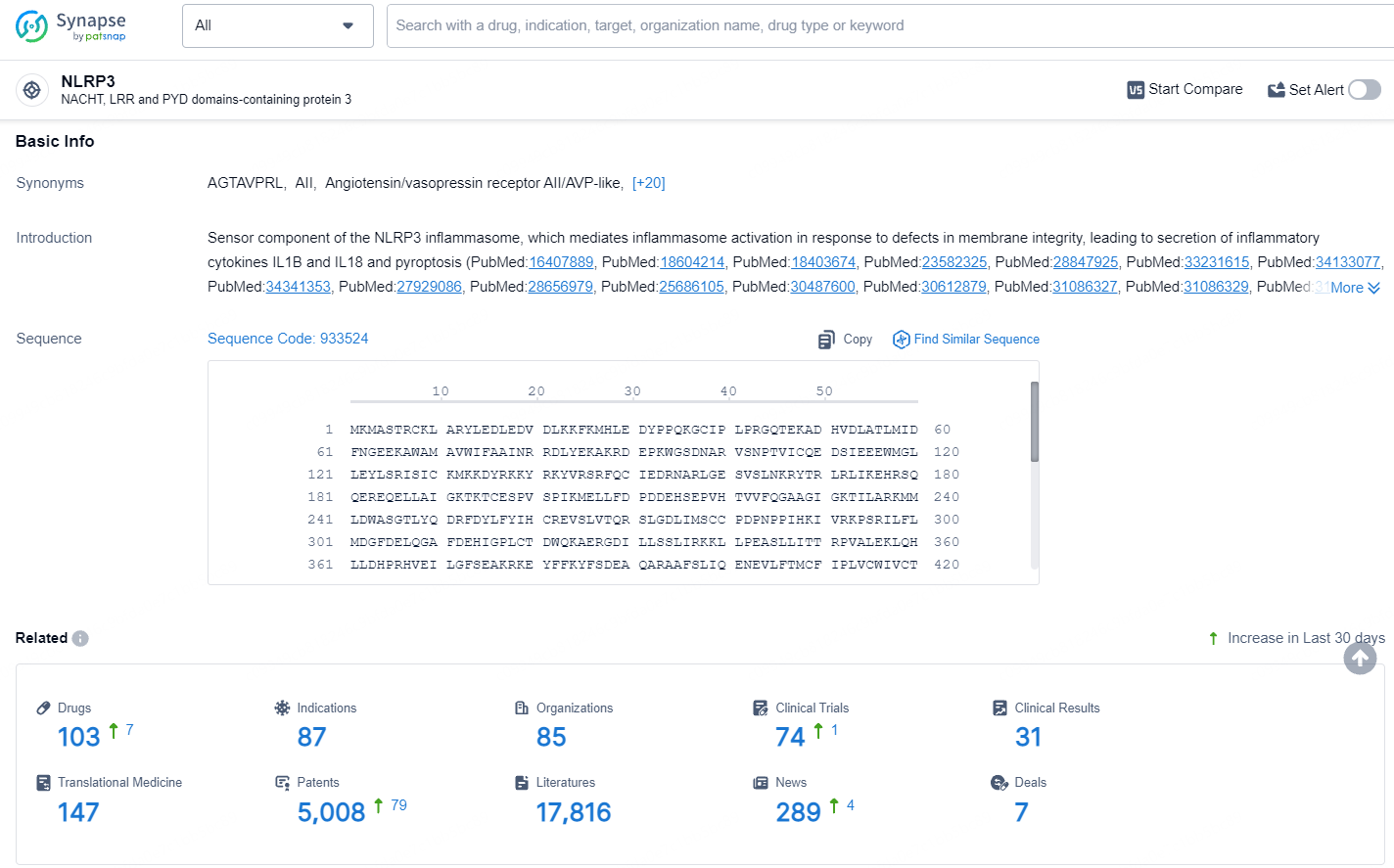
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of July 17, 2024, there are 103 investigational drugs for the NLRP3 target, including 87 indications, 85 R&D institutions involved, with related clinical trials reaching 74, and as many as 5008 patents.
Dapansutrile is in the highest phase of development globally, which is Phase 2/3. This indicates that the drug has shown promising results in preclinical and early clinical trials and is advancing towards potential approval for commercial use. The fact that Dapansutrile targets the NLRP3 protein suggests that it may have anti-inflammatory properties, which could be beneficial in treating various diseases within the specified therapeutic areas.