Vivani Medical Shares New Plans for NPM-115 Small, Long-Term GLP-1 Implant for Obesity
Vivani Medical, Inc., a pioneering company in biopharmaceuticals, revealed plans to commence the inaugural clinical trial for the NPM-115 initiative in Australia, anticipated for the fourth quarter of 2024, subject to securing regulatory approval. The NPM-115 clinical program is set to assess the experimental 6-month GLP-1 implant designed for long-term weight management in individuals who are obese or overweight and have associated comorbidities.
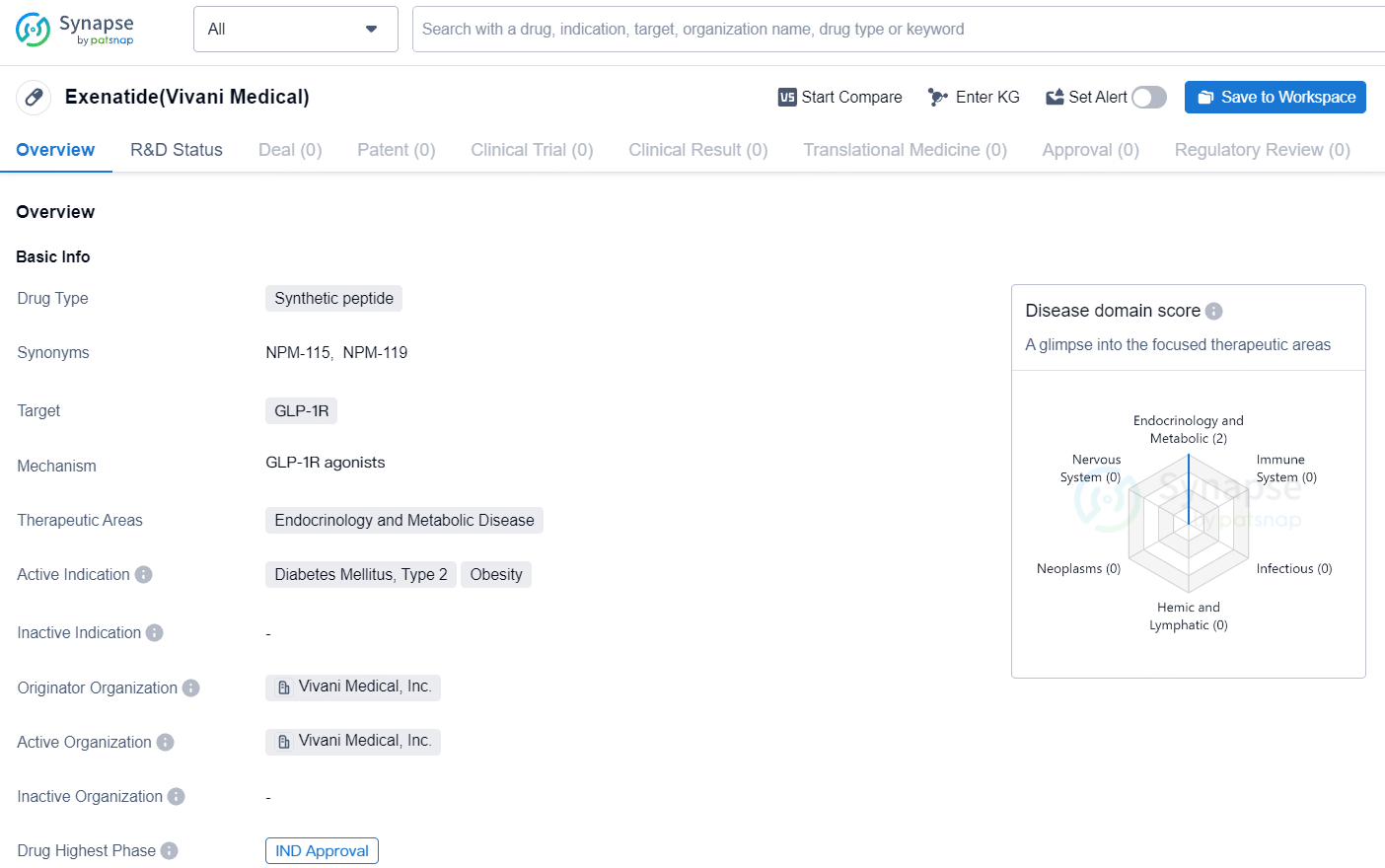
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The findings from LIBERATE-1 might justify the commencement of a follow-up clinical trial, subject to regulatory approval, to examine higher and potentially more efficacious doses of our exenatide implant concerning weight loss and tolerability in overweight or obese individuals.
"In February, we announced a shift in our development priorities for our GLP-1 implants, concentrating on obesity treatment and chronic weight management due to the significant medical necessity and unprecedented market demand," stated Adam Mendelsohn, Ph.D., President and CEO of Vivani.
Once we determine the target dosage, we plan to evaluate an implant at that dosage over a full 6-month period, throughout which our implant has already shown promising outcomes in preclinical animal studies. Moreover, we believe the results from LIBERATE-1 could serve as clinical validation for our NanoPortal drug delivery system, potentially supporting broader use of the technology in treating chronic conditions," noted Adam Mendelsohn.
On February 28, 2024, Vivani disclosed positive preclinical weight loss results from its exenatide implant, bolstering its NPM-115 development initiative and comparable to semaglutide injections. This also marked a strategic decision to prioritize its obesity-related portfolio.
On June 13, 2024, Vivani announced that the U.S. Food and Drug Administration had sanctioned its Investigational New Drug for the exenatide implant to be investigated in patients with type 2 diabetes, supporting the NPM-119 development program.
Previously, the Company had declared an intention to start the first study in the NPM-119 program in the latter half of this year. Given the reallocation of focus towards GLP-1 implants for treating obesity and chronic weight management, the Company now intends to advance the NPM-115 study first. Dr. Mendelsohn mentioned that the NPM-115 program employs the same exenatide implant as NPM-119, with the only difference being the study population.
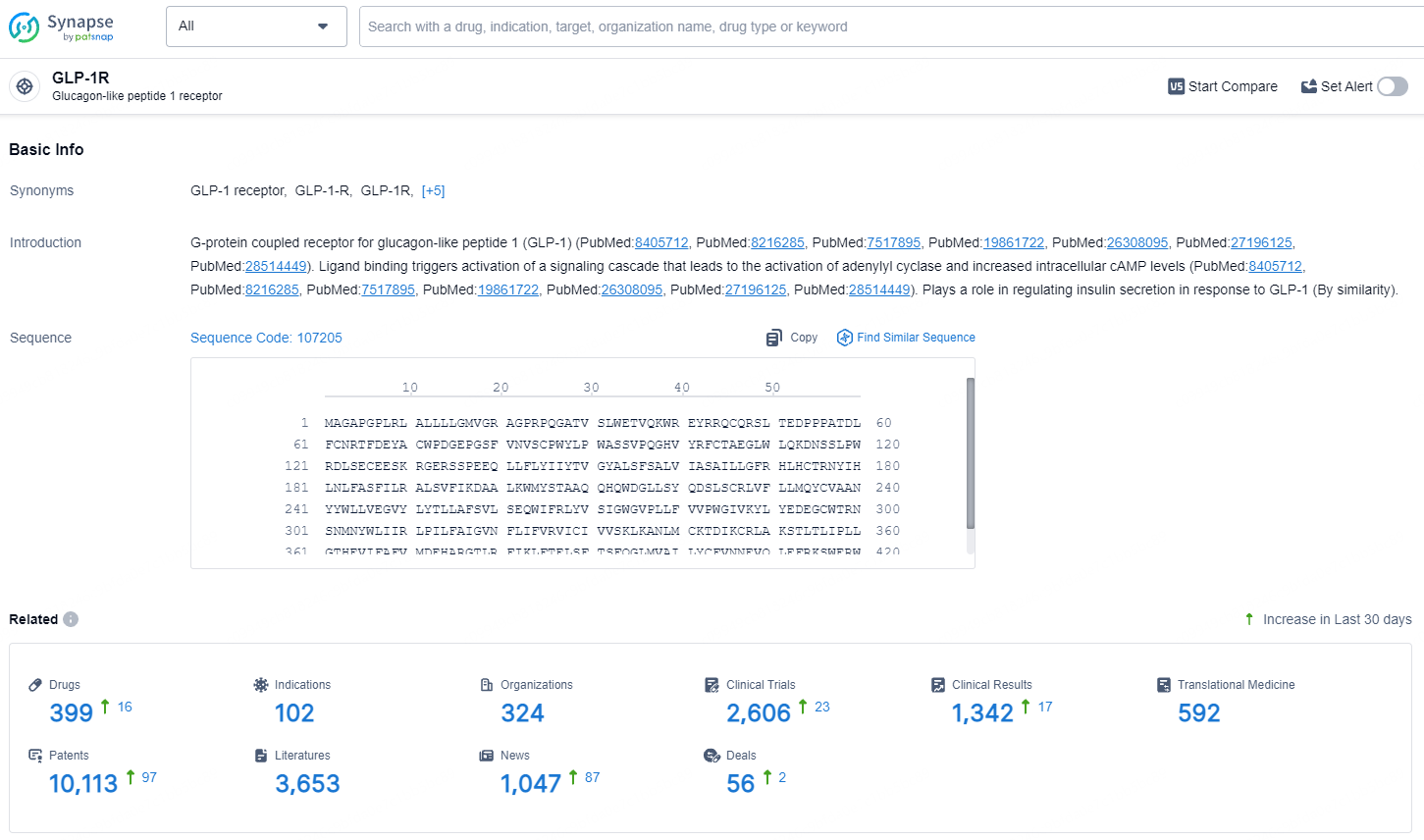
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of July 16, 2024, there are 399 investigational drugs for the GLP-1 targets, including 102 indications, 324 R&D institutions involved, with related clinical trials reaching 2606, and as many as 10113 patents.
Exenatide represents a promising therapeutic option in the field of biomedicine, offering potential benefits for patients with these specific conditions. As the drug advances through clinical trials and potentially towards regulatory approval, it could make a meaningful impact on the treatment landscape for diabetes and obesity.