The initial participant has received a dose in the Phase I clinical study of YOLT-201
YolTech Therapeutics, a biotechnology company focused on in vivo gene editing treatments for rare genetic conditions, has announced the successful enrollment of the inaugural patient in the Phase I clinical trial of YOLT-201, their proprietary in vivo gene editing therapy. This achievement represents a crucial step forward in the clinical progress of this therapeutic candidate.
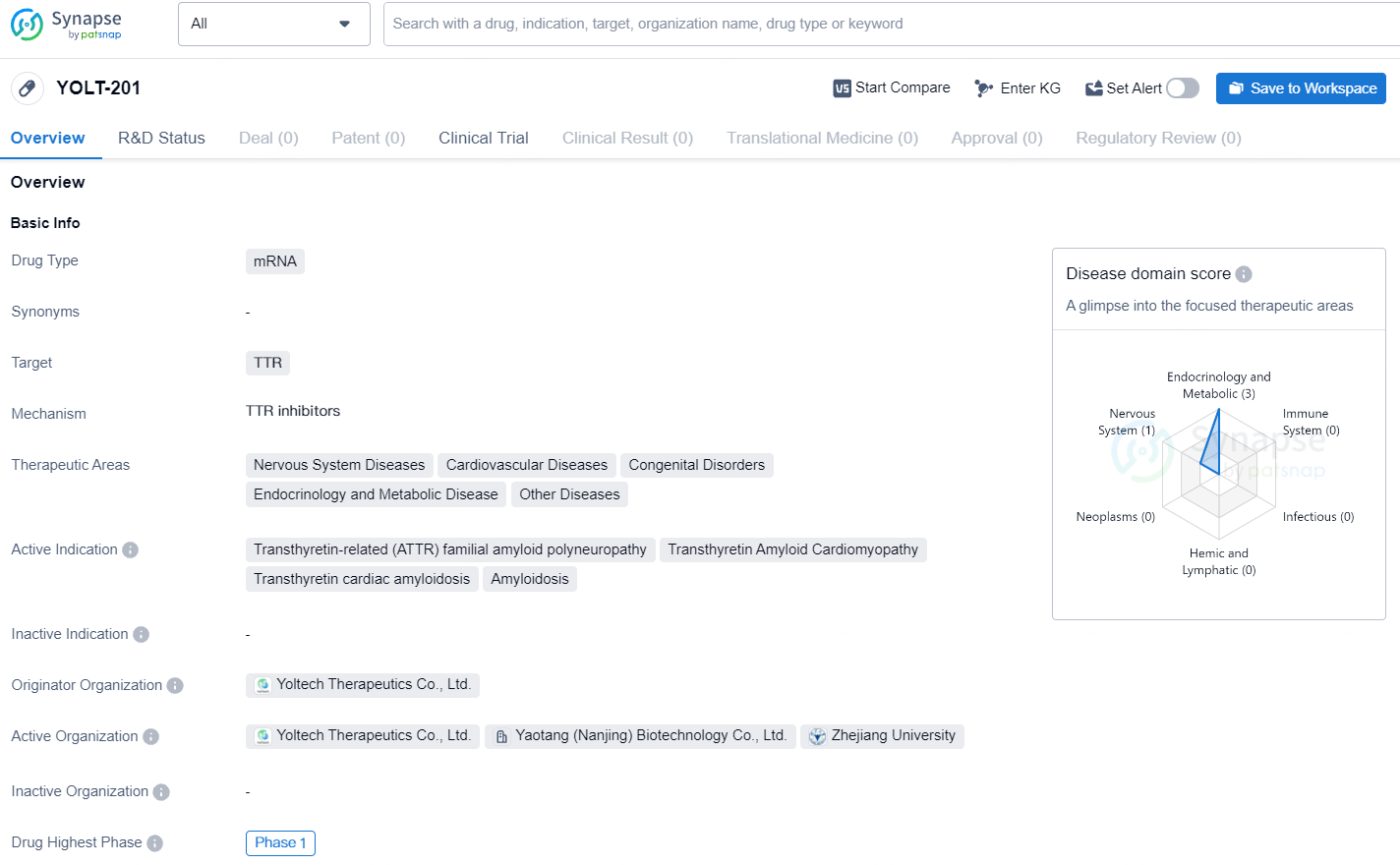
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
ATTR is a severe inherited disorder, resulting from the misfolding of the transthyretin protein (TTR), which leads to the formation of amyloid fibrils that accumulate in various body tissues and organs, including the heart myocardium and peripheral nerves. Depending on the specific mutation, hATTR can present in individuals as young as their teens and twenties, although it is more commonly diagnosed in individuals over the age of 50.
YOLT-201 is a global frontrunner in the advancement of in vivo gene editing therapies and lipid nanoparticle delivery technologies. Preclinical studies in NHP models have demonstrated that a single infusion of YOLT-201 can safely and effectively reduce TTR protein levels in the serum, potentially offering long-lasting therapeutic benefits.
This clinical trial will recruit eligible ATTR patients from across the country. The initial participant received their first dose on June 28, 2024. Two weeks following the dose, the patient remained in good health with no significant adverse effects related to the treatment.
"The completion of the first patient's dosing and safety evaluation marks a crucial step in the clinical progression of YOLT-201," stated Dr. Yuxuan Wu, Founder and CEO of YolTech. "The preliminary clinical trials have shown outstanding safety and efficacy for YOLT-201, and we are optimistic that it can revolutionize the global therapeutic approach to ATTR diseases."
On March 1, 2024, YolTech Biotech revealed that the Center for Drug Evaluation of the National Medical Products Administration had approved the clinical trial application for YOLT-201. This approval makes YOLT-201 the first in vivo gene editing drug delivered via lipid nanoparticles to receive clinical trial authorization in China.
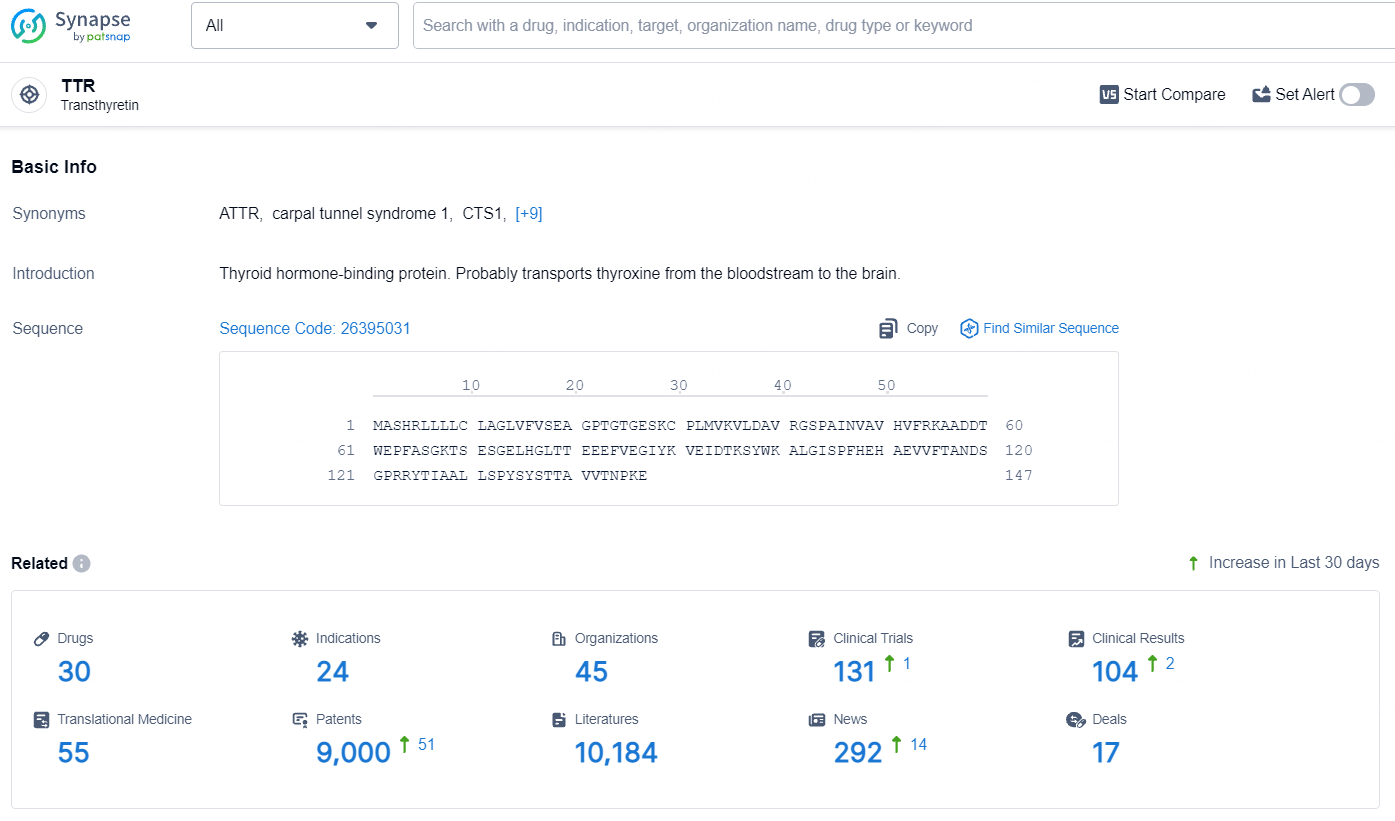
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of July 17, 2024, there are 30 investigational drugs for the TTR target, including 24 indications, 45 R&D institutions involved, with related clinical trials reaching 131, and as many as 9000 patents.
YOLT-201 shows promise in addressing a variety of serious medical conditions related to TTR protein dysfunction. Further advancements in clinical trials and research could potentially lead to the approval and commercialization of this drug for treating a wide range of diseases within the therapeutic areas specified.