Advances in Clinical Research on NMDA Receptor Agonist
The NMDA receptor (N-methyl-D-aspartic acid receptor) is a subtype of the ionotropic glutamate receptor, with a complex molecular structure and unique pharmacological properties. It plays a critical physiological role in the development of the nervous system, such as regulating neuronal survival, regulating the development of neuronal dendrites and axonal structures, and participating in the formation of synaptic plasticity. It also plays a key role in the formation of neuronal circuits. Data suggests that NMDA receptors are a class of receptors that are crucial in the processes of learning and memory.
Activation of the NMDA receptor allows the influx of calcium ions into neurons, which is essential for neuronal communication and the formation of new connections. However, dysregulation of NMDA receptor activity has been implicated in several neurological disorders, including Alzheimer's disease, Parkinson's disease, and epilepsy. Understanding the role of NMDA receptors is vital for developing therapeutic interventions targeting these conditions.
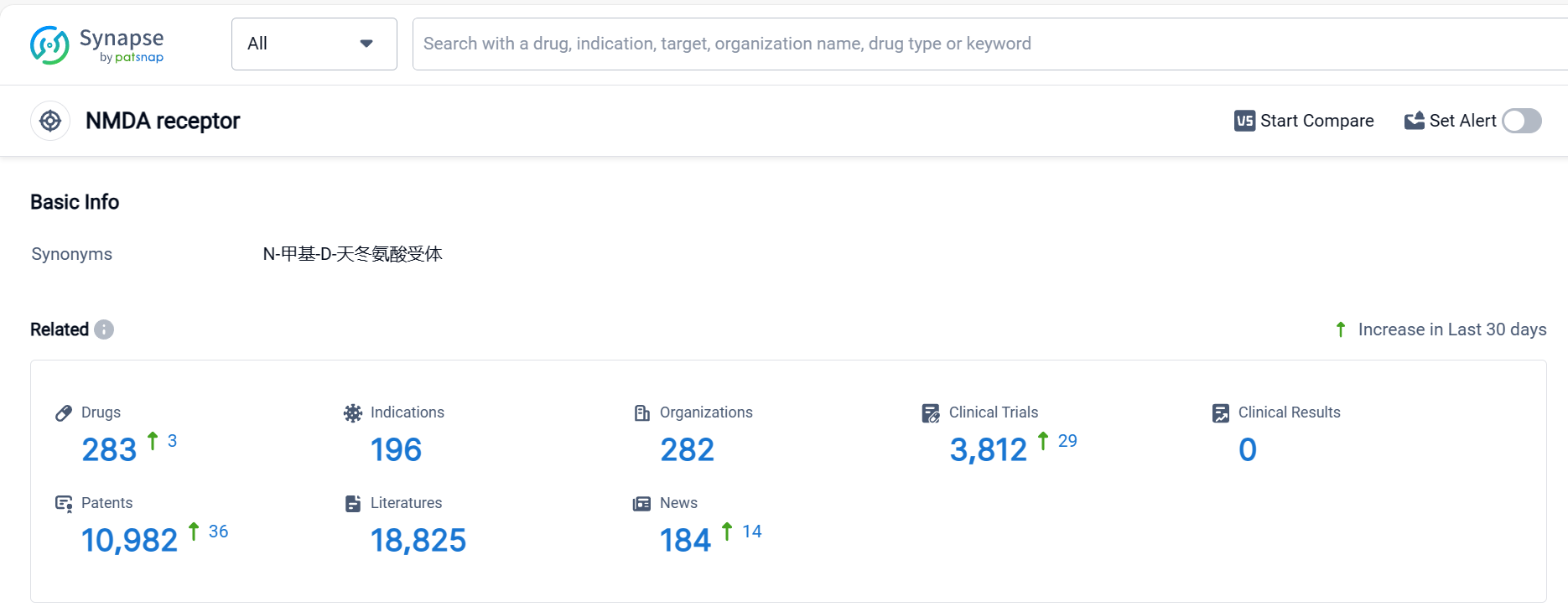
NMDA Receptor Competitive Landscape
According to Patsnap Synapse, as of 5 Oct 2023, there are a total of 283 NMDA receptor drugs worldwide, from 282 organizations, covering 196 indications, and conducting 3812 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
The analysis of the target NMDA receptor reveals a competitive landscape with multiple companies actively developing drugs. The highest stage of development is the "Approved" phase, indicating successful drug development in various indications. Small molecule drugs are progressing most rapidly, with a significant number of approved drugs. China is emerging as a key player in the development of drugs targeting the NMDA receptor. Further analysis of R&D progress, biosimilars, and specific indications is required to provide more detailed insights into the current competitive landscape and future development of the target NMDA receptor.
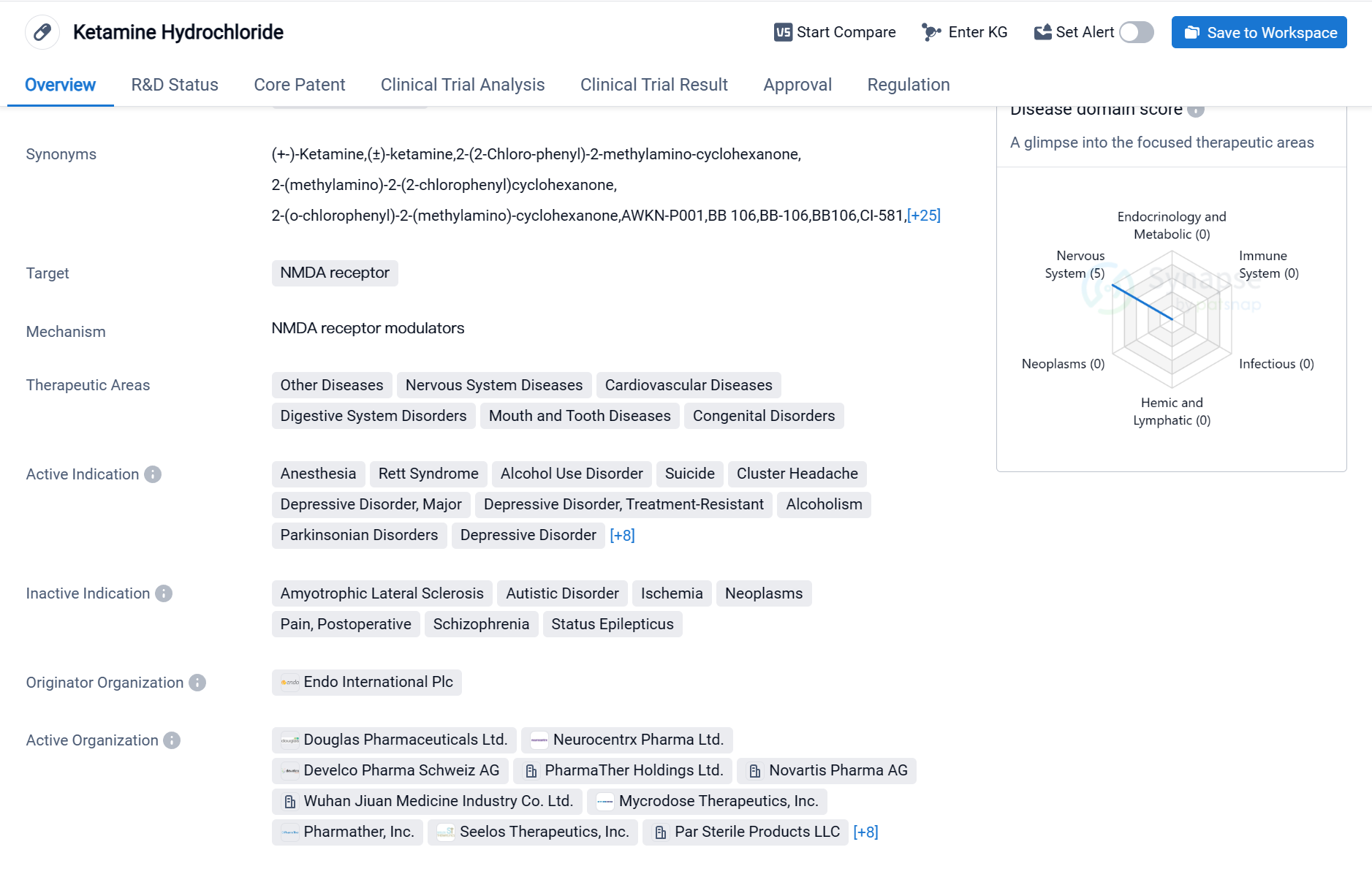
Key drug: Ketamine Hydrochloride
Ketamine Hydrochloride is a small molecule drug that primarily targets the NMDA receptor. It has been approved for various therapeutic areas, including Other Diseases, Nervous System Diseases, Cardiovascular Diseases, Digestive System Disorders, Mouth and Tooth Diseases, and Congenital Disorders. The drug has shown efficacy in treating multiple indications, such as Anesthesia, Rett Syndrome, Alcohol Use Disorder, Suicide, Cluster Headache, Depressive Disorder (Major and Treatment-Resistant), Alcoholism, Parkinsonian Disorders, Bipolar Disorder, Acute Pain, Mucositis, Pain, Stomatitis, Stress Disorders (Post-Traumatic), Reperfusion Injury, and Smoking Cessation.
Ketamine Hydrochloride was first approved in the United States in February 1970 and is currently in the highest phase of approval globally. The drug's originator organization is Endo International Plc. It is regulated under the Innovative Licensing and Access Pathway and has also been designated as an Orphan Drug.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
As a small molecule drug, Ketamine Hydrochloride acts on the NMDA receptor, which plays a crucial role in the transmission of pain signals and the regulation of mood and cognition. Its approval for various therapeutic areas highlights its potential in addressing a wide range of diseases and disorders.
The drug's approval for anesthesia indicates its effectiveness in inducing and maintaining a state of unconsciousness during surgical procedures. Additionally, Ketamine Hydrochloride has shown promise in the treatment of Rett Syndrome, a rare genetic disorder that affects brain development and causes severe cognitive and physical impairments.
Furthermore, the drug has been studied for its potential in managing Alcohol Use Disorder, Suicide, Cluster Headache, and various depressive disorders, including major depression and treatment-resistant depression. Its ability to modulate the NMDA receptor may contribute to its antidepressant effects.
Ketamine Hydrochloride's approval for alcoholism and smoking cessation suggests its potential in addressing addictive behaviors. It has also been explored for its analgesic properties in acute pain, mucositis, stomatitis, and post-traumatic stress disorders.
Overall, Ketamine Hydrochloride's wide range of approved indications and its targeting of the NMDA receptor make it a versatile drug with potential applications in various therapeutic areas. Its long history of approval since 1970 demonstrates its established safety and efficacy profile.