The Magic Bullets of Cancer Treatment: ADCs and Their Journey from Concept to Clinic
On July 10, BeiGene and DualityBio have announced an agreement for BeiGene to acquire an exclusive option for a global clinical and commercial license to an investigational ADC therapy for patients with select solid tumors. DualityBio will continue preclinical research activities and support Investigational New Drug filing while BeiGene will hold global clinical, manufacturing, and commercial rights upon exercising its option. Under the terms of the agreement, DualityBio will receive an upfront payment and will be eligible for additional payments based on certain development, regulatory, and commercial milestones, totaling up to $1.3 billion, in addition to tiered royalties. The partnership aims to accelerate the development of this asset and bring more breakthrough ADC medicines to patients worldwide.
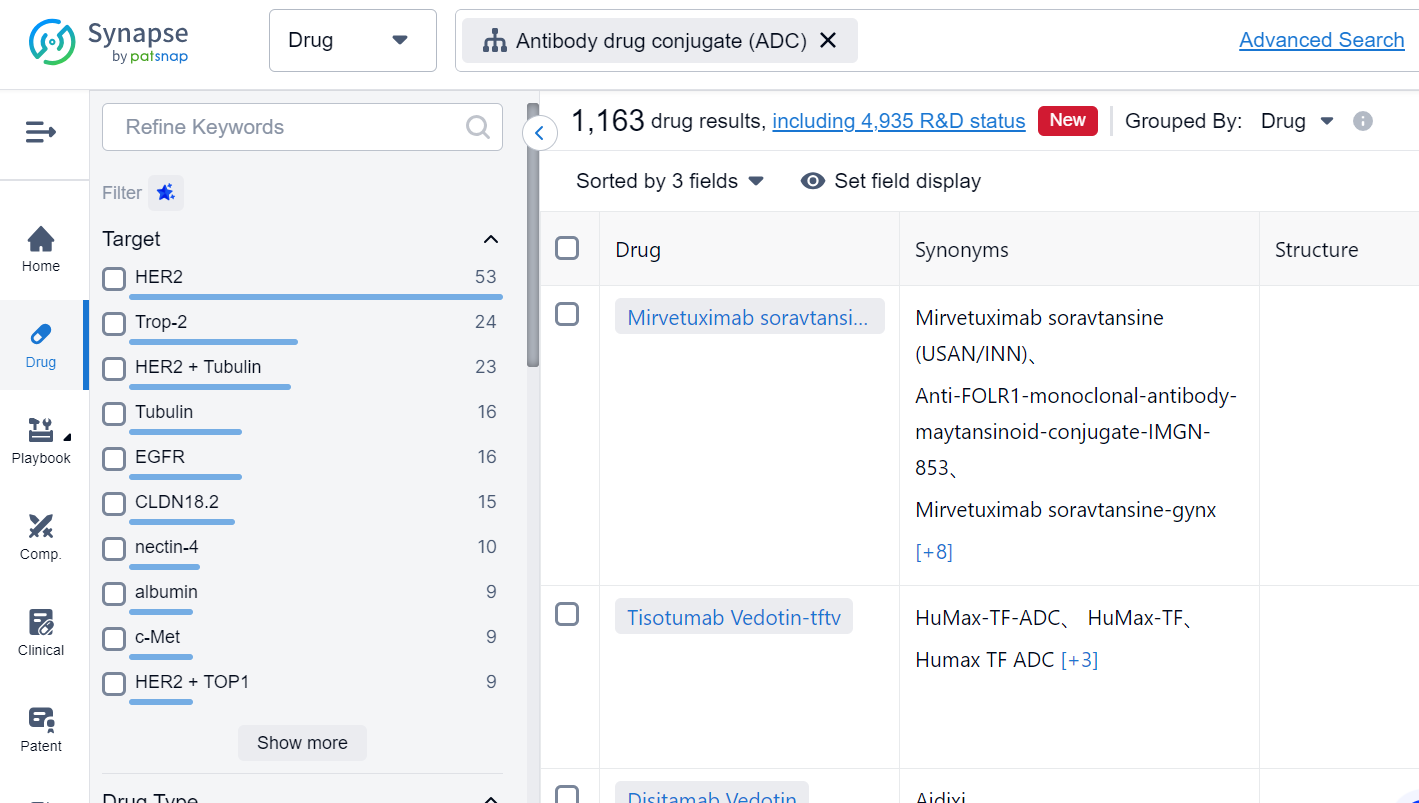
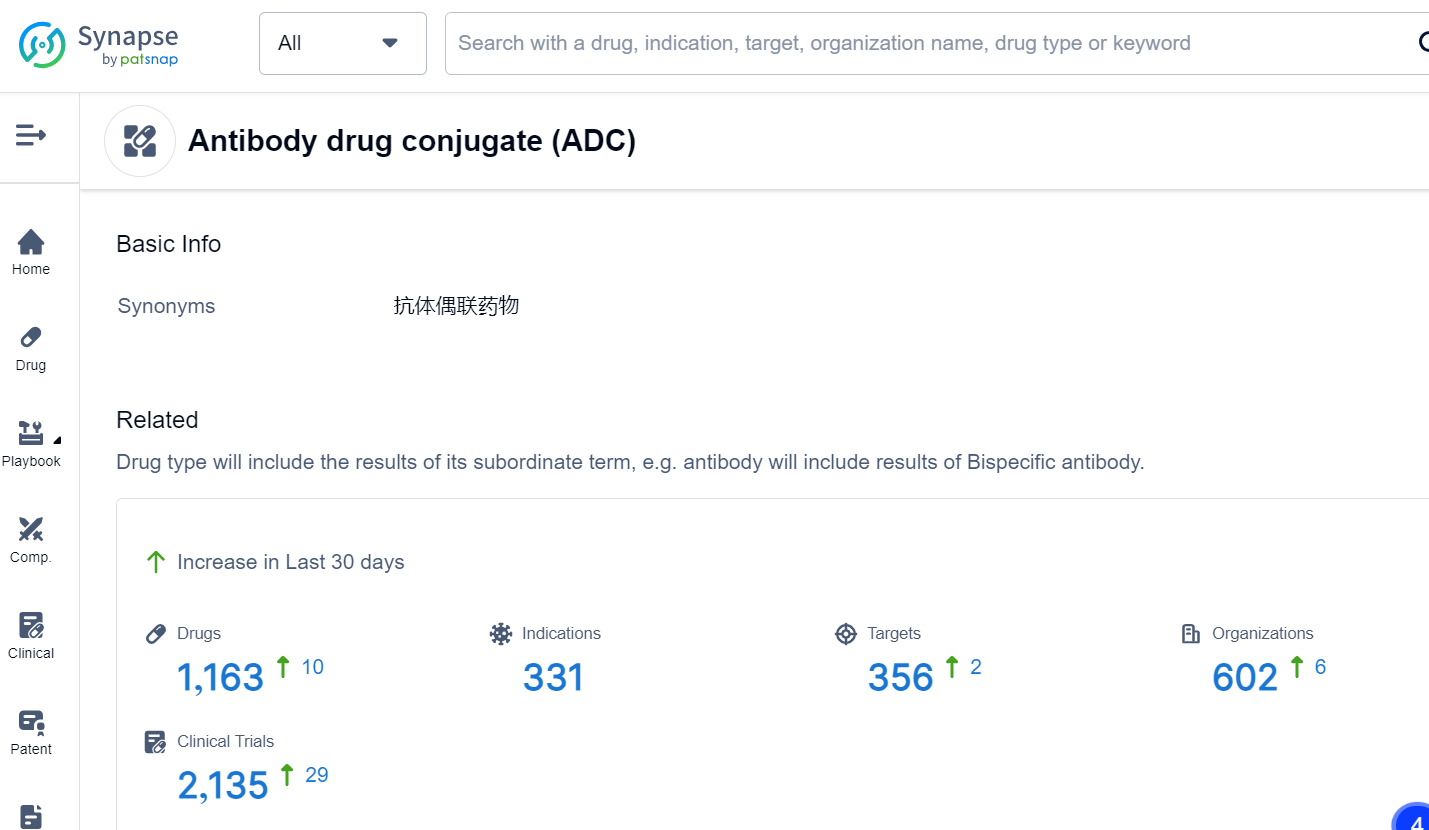
This is the latest move by the biotech industry to jump on the ADC bandwagon. Antibody drug conjugates (ADCs) are a class of biopharmaceutical drugs designed as a targeted therapy for treating cancer. Unlike conventional chemotherapy, which can damage healthy cells, ADCs are intended to target and kill tumor cells while sparing healthy cells. In the Synapse database, there are already 1,163 drugs that fall into this category, with a variety of targets. For the latest information on ADC drugs in development, simply click the image below to register for free access to Synapse's vast dataset:
The concept of ADCs originated from Paul Ehrlich's work in 1900. He envisioned drugs that act like a “magic bullet” by selectively delivering toxic agents to microbes or tumor cells.
The first successful ADC clinical trial occurred in 1983, using an anti-carcinoembryonic antigen antibody conjugated to vindesine. In the 1990s, the first generation of ADCs used murine antibodies as a backbone. However, the linkers were unstable in human circulation and ADCs had a short half-life.
In 2000, the FDA approved the first ADC: gemtuzumab ozogamicin (trade name Mylotarg), targeting CD33-positive acute myeloid leukemia. It used calicheamicin as the cytotoxic payload linked by a hydrazone linker. However, it was withdrawn in 2010 due to severe hepatotoxicity and lack of benefit. It was reintroduced in 2017 at a lower dose and different schedule.
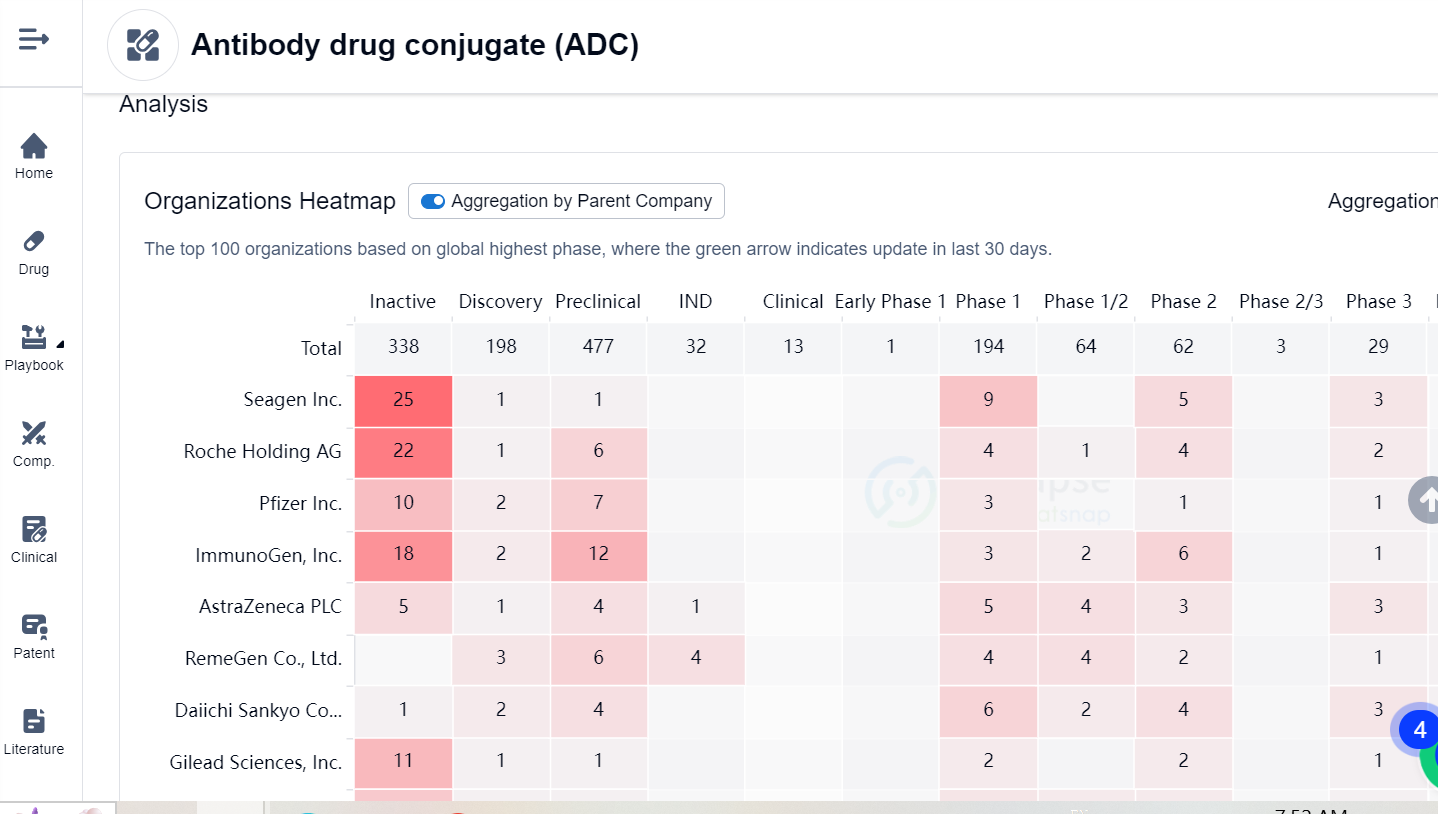
Since then, several ADCs have been approved for various cancers like Hodgkin lymphoma, breast cancer, urothelial cancer, and multiple myeloma. These ADCs employ different antibodies, linkers and payloads to achieve better efficacy and safety. Examples include brentuximab vedotin (Adcetris), trastuzumab emtansine (Kadcyla), inotuzumab ozogamicin (Besponsa), and belantamab mafodotin (Blenrep). For more information, Synapse’s Competition Landscape analysis provides a heatmap of top 100 organizations based on global highest phase in ADC drugs:
In the rapidly evolving field of cancer research, the development of ADCs comes with its unique set of challenges. To start, the selection of the target antigen is crucial. This antigen should ideally be highly expressed on tumor cells and minimally expressed on normal cells to alleviate toxicity risk. This antigen should also enable internalization upon antibody binding to directly facilitate payload delivery, and be stable enough not to be affected by the tumor microenvironment or therapy measures.
Next, antibody engineering is paramount. It requires high specificity and affinity for the target antigen, along with favorable pharmacokinetics and biodistribution. These antibodies should also demonstrate minimal adverse effects like immunogenicity, cytokine release, or compliment activation. Besides, provisions for an optimal linker and payload attachment might require a suitable conjugation site and drug-to-antibody ratio (DAR).
Linker design also poses hurdles. It necessitates a balance between stability in the bloodstream and cleavability in the tumor cell to allow active payload release. Consideration towards the linker's length, flexibility, toxicity, and immunogenicity is critical for ADC's conformations and functions.
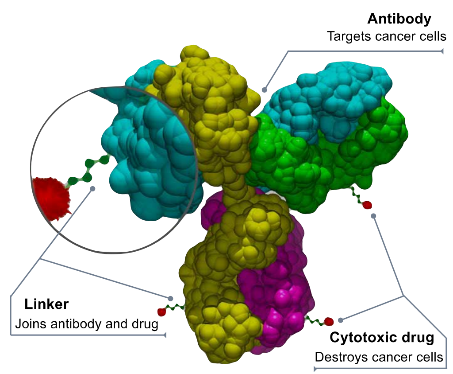
Payload selection is another concern. These payloads should be potent and efficacious against tumor cells with minimal off-target toxicity, resistance, and optimal solubility for such conjugation and delivery.
Manufacturing poses an additional challenge. ADCs production is a complex, costly process that demands tight control over aspects like conjugation reaction, purification, formulation, and storage. The quality is influenced by factors like aggregation, degradation, heterogeneity, impurities, and stability.
Lastly, the clinical development of ADCs is demanding due to dose selection, patient selection, biomarker identification, safety monitoring, and efficacy evaluation. Regulatory hurdles, such as the need for novel endpoints, surrogate markers, combination strategies, and post-marketing studies, also pose significant barriers.
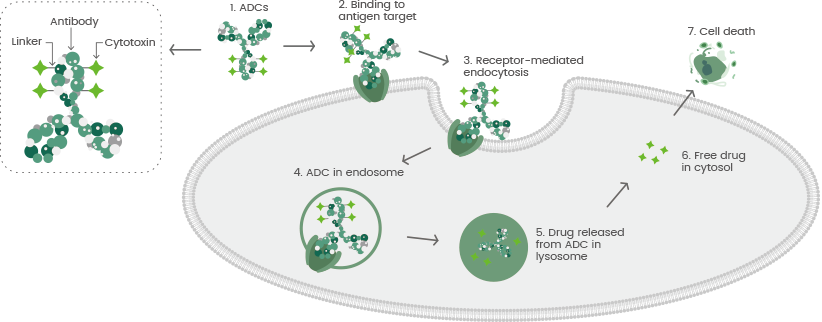
Despite these formidable obstacles, the potential benefits of ADCs are immense, making their development a vital element in contemporary cancer research and treatment.
Emerging directions for ADC development are eagerly being explored. One key area of focus revolves around exploiting new target antigens, such as cell surface receptors, enzymes, transporters, or glycoproteins, offering novel opportunities for selective targeting of tumor cells and their microenvironments.
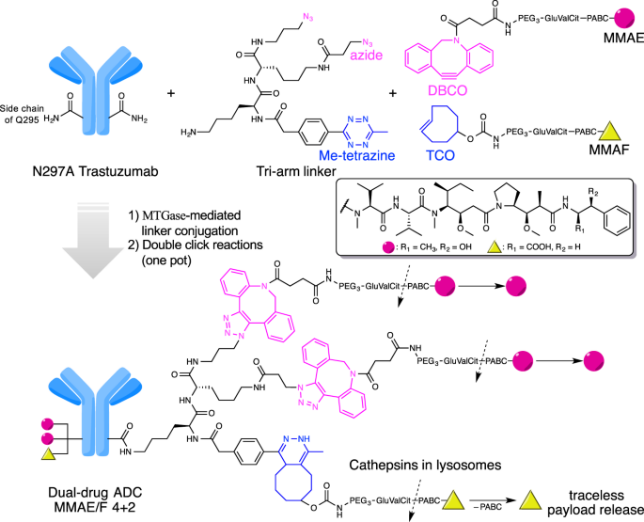
Efforts are also being made to increase the drug-to-antibody ratio (DAR), expected to enhance ADCs' potency by delivering more payload molecules per antibody. This may be achieved by using site-specific conjugation methods, multivalent antibodies, or even polymer-based carriers.
Increasing antibody penetration into solid tumors, through the use of smaller antibody fragments, bispecific antibodies, or engineered antibodies, is another intriguing perspective, which could contribute to better distribution of ADCs within tumor tissues.
Search for strategies to combat resistance mechanisms conferred to ADCs, such as reduced antigen expression levels or payload resistance, is also underway. The use of combination therapies, alternative payloads, or novel linkers may be decisive in this field.
Enhancing the efficiency of ADC uptake and processing by tumor cells is a promising avenue where pH-sensitive linkers, enzyme-responsive linkers, or self-immolative linkers may lead to better payload activation within tumor cells.
Contending with off-target payload exposure is a crucial issue, and methods such as masked payloads, prodrug payloads, or targeted delivery systems are being considered to reduce the systemic toxicity and adverse effects associated with ADCs.
Finally, the employment of non-cytotoxic payloads - like immunomodulators, gene silencers, or radioligands - opens up an exciting area of exploration that may introduce new modes of action for ADCs.
Despite the complexity and multiplicity of challenges, the future of ADCs appears promising, wielding substantial potential for improving cancer treatment outcomes.