Pharmaceutical Insights: Clemastine Fumarate's R&D Progress and its Mechanism of Action on Drug Target
Clemastine Fumarate's R&D Progress
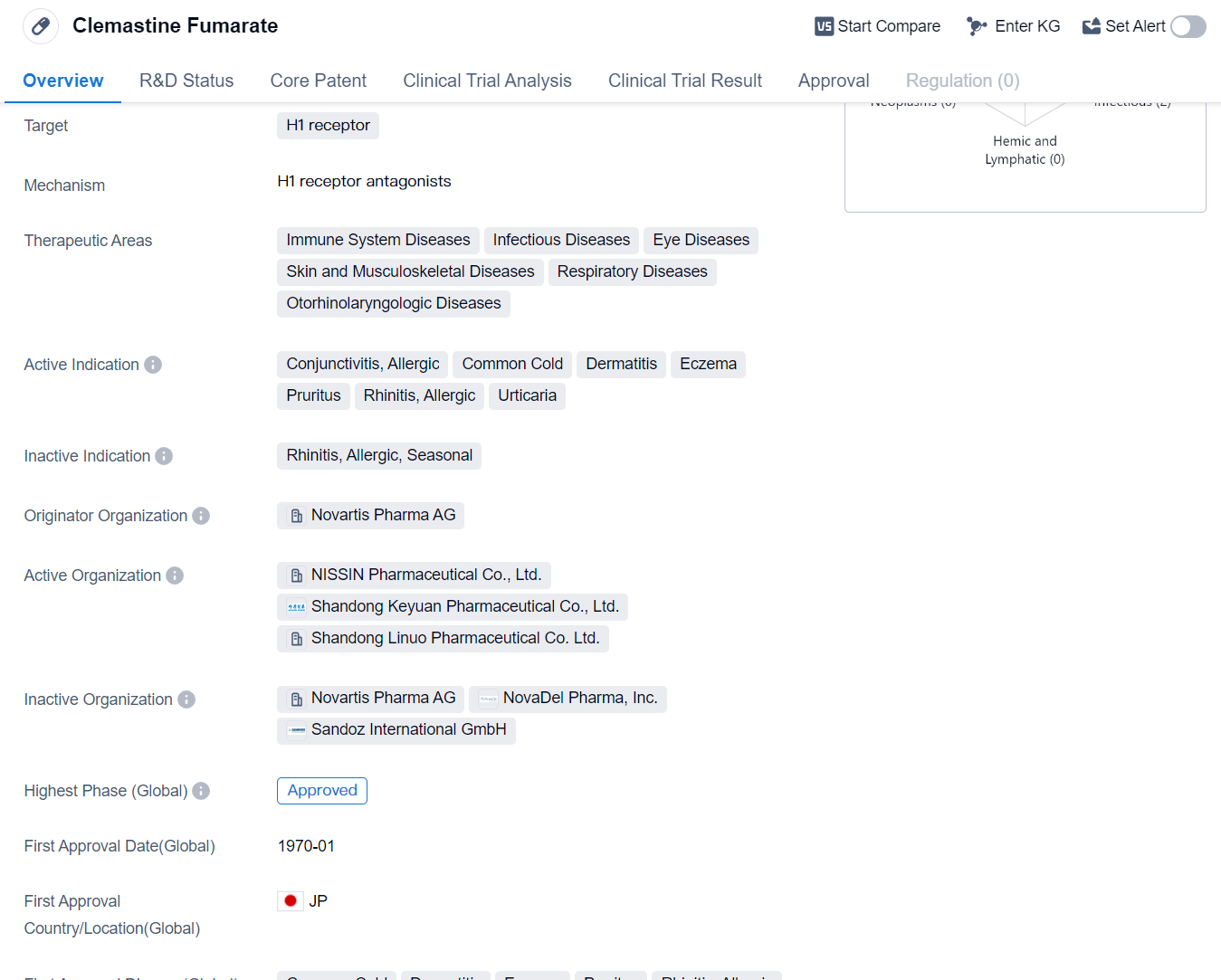
Clemastine Fumarate is a small molecule drug that primarily targets the H1 receptor. It has been approved for use in various therapeutic areas, including immune system diseases, infectious diseases, eye diseases, skin and musculoskeletal diseases, respiratory diseases, and otorhinolaryngologic diseases. The drug is indicated for the treatment of conjunctivitis, allergic common cold, dermatitis, eczema, pruritus, allergic rhinitis, and urticaria.
The originator organization of Clemastine Fumarate is Novartis Pharma AG, a renowned pharmaceutical company. The drug has reached the highest phase of development which is approved globally. Its first approval date in the global market was in January 1970, with Japan being the first country to approve its use.
Clemastine Fumarate is primarily used to alleviate symptoms associated with various allergic conditions. Conjunctivitis, commonly known as pink eye, is an inflammation of the conjunctiva, the thin tissue covering the white part of the eye. Allergic conjunctivitis occurs when the conjunctiva becomes irritated due to an allergic reaction. Clemastine Fumarate can help relieve the symptoms of allergic conjunctivitis, such as redness, itching, and watering of the eyes.
The drug is also effective in treating the common cold, a viral infection that affects the upper respiratory tract. It can help alleviate symptoms like a runny or stuffy nose, sneezing, and nasal congestion. Additionally, Clemastine Fumarate is used to manage dermatitis and eczema, both of which are inflammatory skin conditions characterized by redness, itching, and rash.
Pruritus, or itching, is another indication for which Clemastine Fumarate is prescribed. It can provide relief from itching caused by various allergic reactions or skin conditions. Allergic rhinitis, commonly known as hay fever, is another condition that can be managed with this drug. It helps alleviate symptoms like sneezing, itching, and a runny or stuffy nose.
Lastly, Clemastine Fumarate is used to treat urticaria, also known as hives. Urticaria is characterized by raised, itchy, and red welts on the skin, often caused by an allergic reaction.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Clemastine Fumarate: H1 receptor antagonist
H1 receptor antagonists, also known as H1 antihistamines, are a class of drugs that block the action of histamine at the H1 receptors. Histamine is a chemical released by the body during an allergic reaction, causing symptoms such as itching, sneezing, runny nose, and watery eyes. H1 receptor antagonists work by binding to the H1 receptors and preventing histamine from attaching to them, thereby reducing or preventing allergic symptoms.
From a biomedical perspective, H1 receptor antagonists are commonly used in the treatment of allergic conditions, such as allergic rhinitis (hay fever), urticaria (hives), and allergic conjunctivitis. They can also be used to manage symptoms of non-allergic conditions, such as motion sickness and insomnia.
There are different generations of H1 receptor antagonists, with each generation having varying degrees of sedative effects. First-generation H1 antihistamines, such as diphenhydramine and chlorpheniramine, are known to cause drowsiness and are often used as sleep aids. Second-generation H1 antihistamines, such as cetirizine and loratadine, have less sedative effects and are preferred for daytime use.
Overall, H1 receptor antagonists are important medications in the management of allergic conditions and provide relief from symptoms associated with histamine release.
Drug Target R&D Trends for Clemastine Fumarate
The analysis of the target H1 receptor in the pharmaceutical industry reveals a competitive landscape with multiple companies growing rapidly. GSK Plc, Sanofi, and Bayer AG have the highest stage of development on this target. The indications with the highest number of approved drugs include rhinitis, allergic, and the common cold. Small molecule drugs are progressing most rapidly, indicating intense competition and potential biosimilar competition. China, the United States, Japan, and the European Union are developing fastest under the current targets, with China showing significant progress.
The current competitive landscape suggests a focus on addressing symptoms and conditions associated with the target H1 receptor, particularly rhinitis, allergic, and the common cold. The future development of the target H1 receptor is likely to involve further advancements in small molecule drugs and potential competition from biosimilars. The progress in China indicates the potential for further growth and innovation in the pharmaceutical industry.
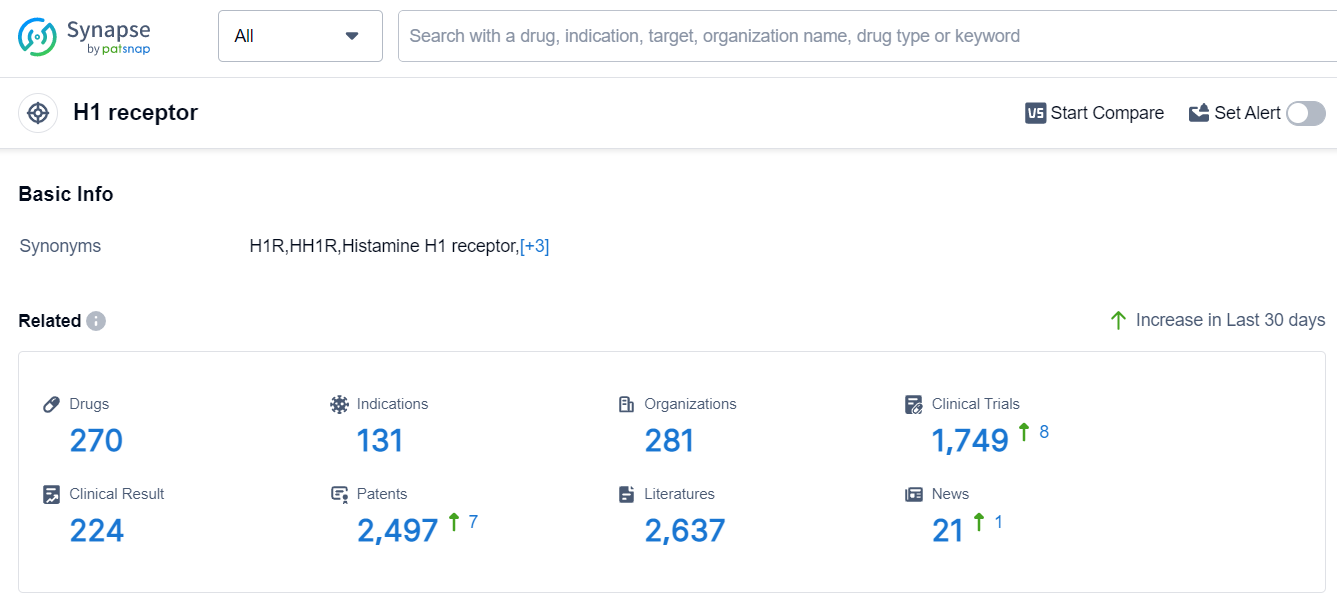
According to Patsnap Synapse, as of 17 Sep 2023, there are a total of 270 H1 receptor drugs worldwide, from 281 organizations, covering 131 indications, and conducting 1749 clinical trials.
Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Clemastine Fumarate is a small molecule drug that targets the H1 receptor and is approved for use in various therapeutic areas. It effectively treats conditions such as conjunctivitis, allergic rhinitis, dermatitis, eczema, pruritus, and urticaria. Its originator organization is Novartis Pharma AG, and it received its first approval in Japan in 1970.