The new indication for Sugemalimab has been approved in China, making it the world's first treatment for R/R ENKTL
Recently, Pfizer announced that the third adaptation of the PD-L1 monoclonal antibody Sugemalimab has been conditionally approved by the National Medical Products Administration (NMPA) of China. The single medicine is used for the treatment of adult patients with recurrent or refractory extranodal NK/T cell lymphoma (R/R ENKTL). As the world's first PD-L1 monoclonal antibody for the treatment of R/R ENKTL, Sugemalimab is expected to change the clinical dilemma in the R/R ENKTL field where there is no standard therapy method.
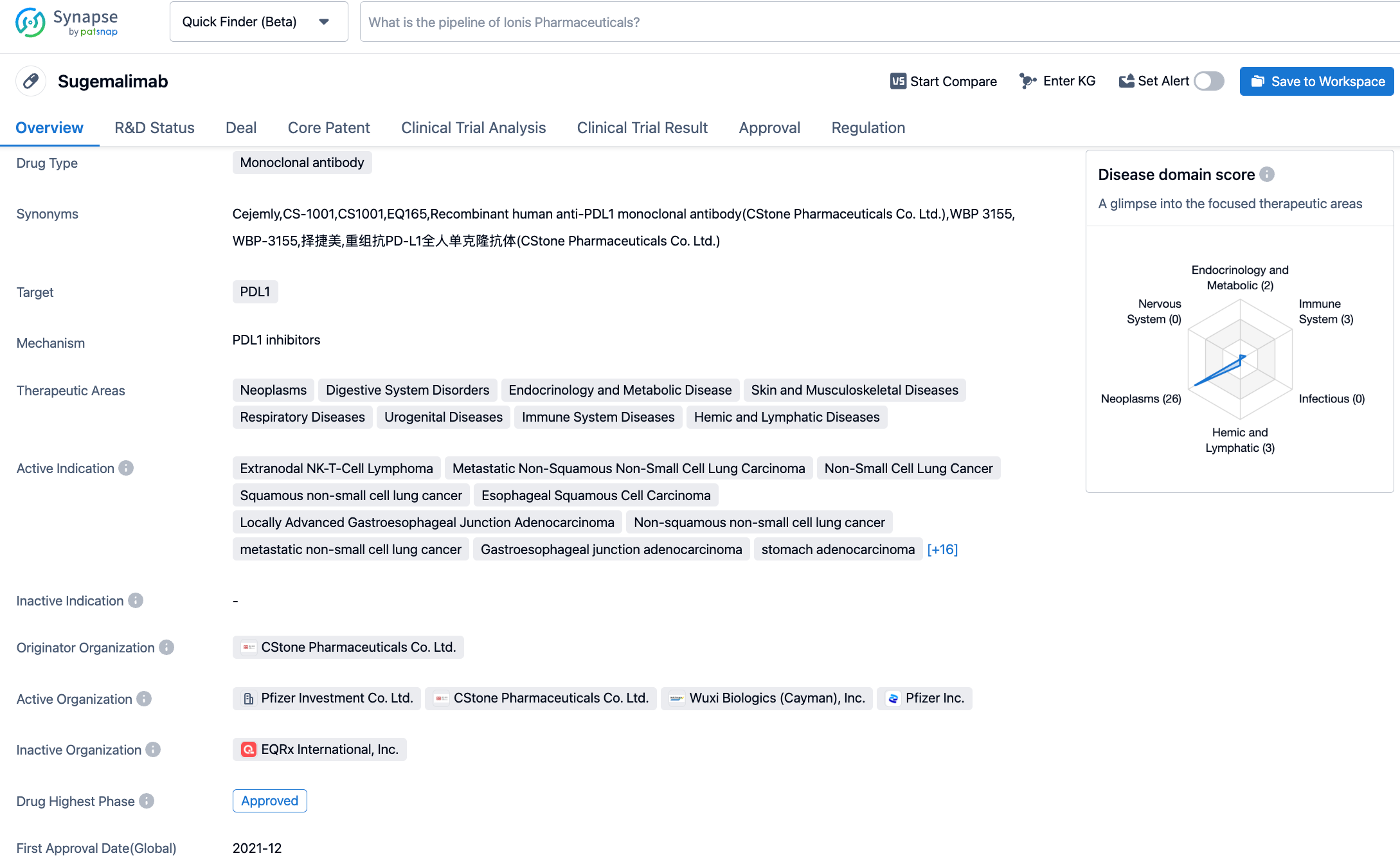
Sugemalimab is an anti-PD-L1 monoclonal antibody developed by CStone Pharmaceuticals. So far, Sugemalimab has been approved in China for two indications: in combination with Pemetrexed and Carboplatin for the first-line treatment of patients with epidermal growth factor receptor (EGFR) gene mutation-negative and anaplastic lymphoma kinase (ALK) negative non-squamous non-small cell lung cancer, and in combination with paclitaxel and carboplatin for the first-line treatment of patients with metastatic squamous non-small cell lung cancer (December 2021). Further, it is also approved for consolidation therapy in patients with unresectable stage III non-small cell lung cancer (NSCLC) who did not progress after concurrent or sequential radiochemotherapy (May 2022).
The approval of this indication for Sugemalimab is based on the GEMSTONE-201 study, which aimed to evaluate its effectiveness and safety as a single drug treatment for adult R/R ENKTL. The study results showed that compared to the historical control, Sugemalimab significantly improved the objective remission rate (ORR). Among the 78 patients assessable for efficacy, the ORR assessed by the Independent Radiological Review Committee (IRRC) was 44.9%, with a complete remission rate of 35.9%. The investigator's objective remission rate assessment was highly consistent with the IRRC assessment. Meanwhile, subgroup analysis suggested that Sugemalimab is broadly effective in patients with R/R ENKTL, including those who have received multiple lines of therapy, regardless of whether they achieved remission in the previous line. Sugemalimab demonstrated good tolerability and safety in patients with R/R ENKTL, with no new safety signals detected. The primary results of this study were presented at the American Society of Clinical Oncology (ASCO) annual meeting in June 2022 and were published in the Journal of Clinical Oncology (JCO) in March 2023.
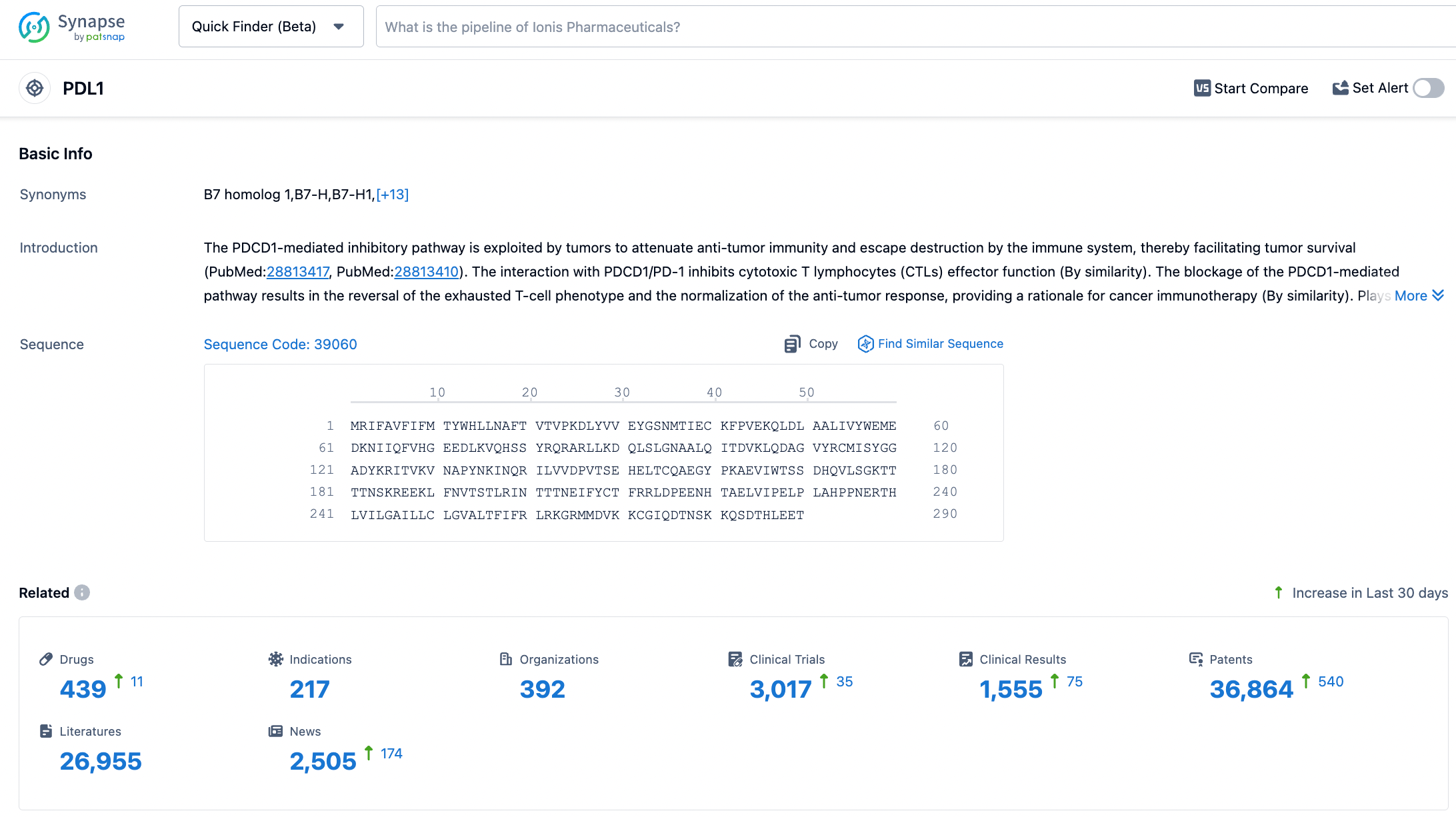
According to the Synapse database, as of November 1, 2023, there are 438 drugs under investigation targeting PD-L1 with 217 different indications, involving 392 institutions, with 3004 related clinical trial findings and 36,685 patents. In October 2020, CStone Pharmaceuticals licensed the development and commercialization rights for Sugemalimab and anti-PD-1 monoclonal antibody CS1003 outside of Greater China to EQRx for up to $1.3 billion. EQRx has submitted applications to the Medicines and Healthcare Products Regulatory Agency (MHRA) and the European Medicines Agency (EMA) for the first-line treatment of metastatic NSCLC with Sugemalimab in combination with chemotherapy, marking the potential for Sugemalimab's successful market entry on a global scale.