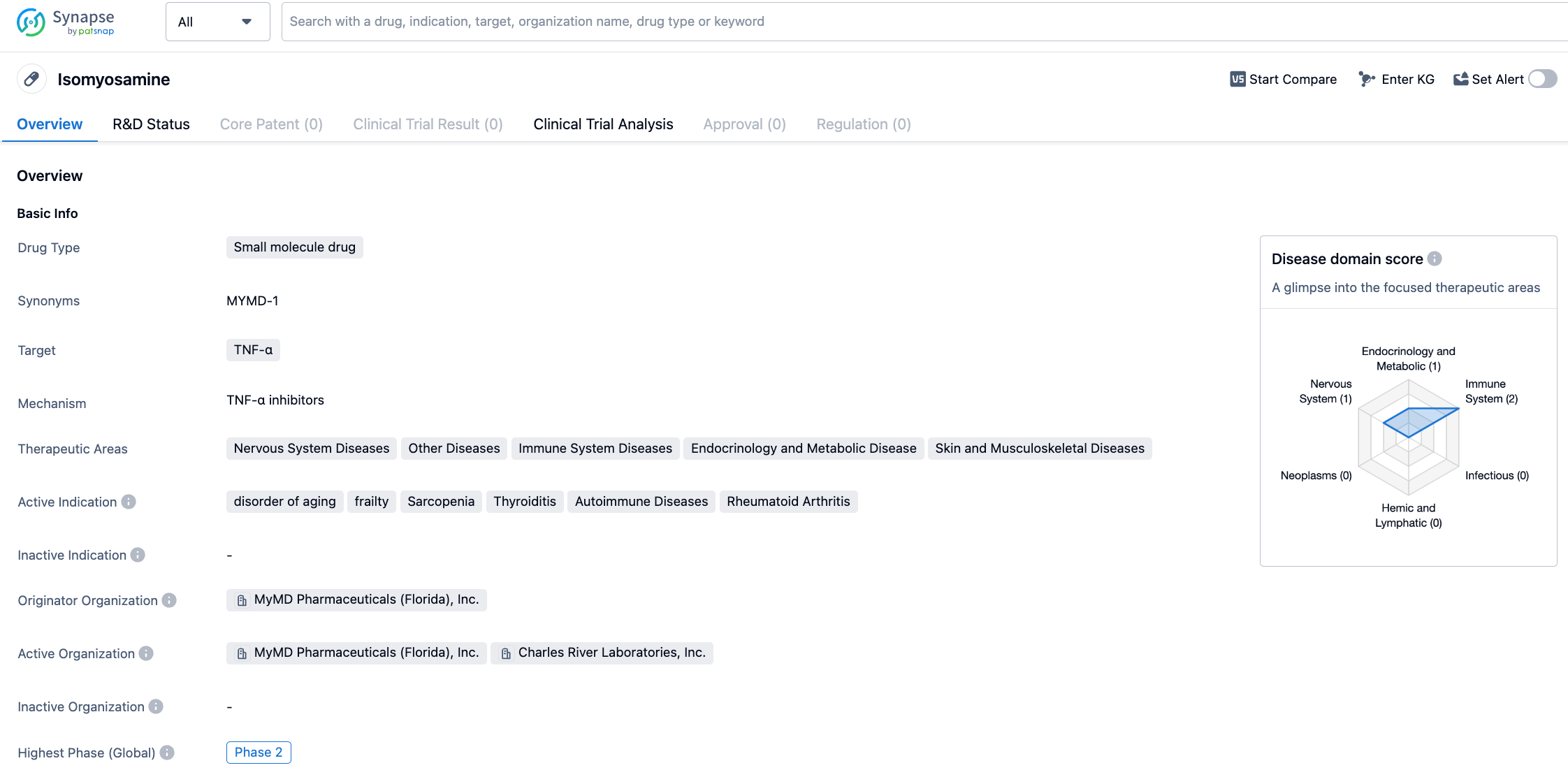
The oral TNF-α drug MYMD-1 of MyMD Pharmaceuticals has reached phase 2 clinical end points
On August 1, 2023, MyMD Pharmaceuticals announced that the oral Tumor Necrosis Factor-α (TNF-α) inhibitor MYMD-1 achieved statistically significant positive results in a Phase 2 randomized clinical trial (NCT05283486). In patients with chronic inflammation caused by sarcopenia or age-related frailty, MYMD-1 met the primary endpoint of significantly reducing biomarkers of chronic inflammation in trial participants. There is potential for MYMD-1 to be the first drug approved by the US FDA for sarcopenia treatment.
MYMD-1, developed by MyMD Pharmaceuticals, is a selective TNF-α inhibitor, a driving factor of chronic inflammation. Research shows that MYMD-1 can delay the aging process, prevent sarcopenia and frailty, and extend healthy lifespans. MYMD-1 has demonstrated effectiveness in regulating the immune system in pre-clinical and clinical studies. It selectively blocks TNF-α when the signaling pathway is over-activated (such as in autoimmune diseases and cytokine storms), but does not block the normal mechanism of TNF-α's reaction to infections.
The study was a multi-center, randomized, double-blind, placebo-controlled clinical trial designed to evaluate the efficacy, tolerability, and pharmacokinetics of MYMD-1 (600mg, 750mg, 900mg, 1050mg) in treating chronic inflammation caused by sarcopenia or frailty. The primary endpoints of the study were 1) serum levels of 3 biomarkers of chronic inflammation (TNF-α, sTNFR1, and IL-6) and 2) pharmacokinetic parameters. The results showed a significant reduction in patients' levels of TNF-α (P=0.008), sTNFR1 (P=0.02), and IL-6 (P=0.03), and MYMD-1 demonstrated appropriate plasma concentrations and pharmacokinetic parameters. Additionally, MYMD-1 displayed good safety and tolerability, with no reported treatment-related adverse events (TRAEs) or serious adverse events (SAEs) during the study period. Based on these positive results, MyMD Pharmaceuticals plans to discuss a Phase III study plan for MYMD-1's treatment of sarcopenia with the FDA.
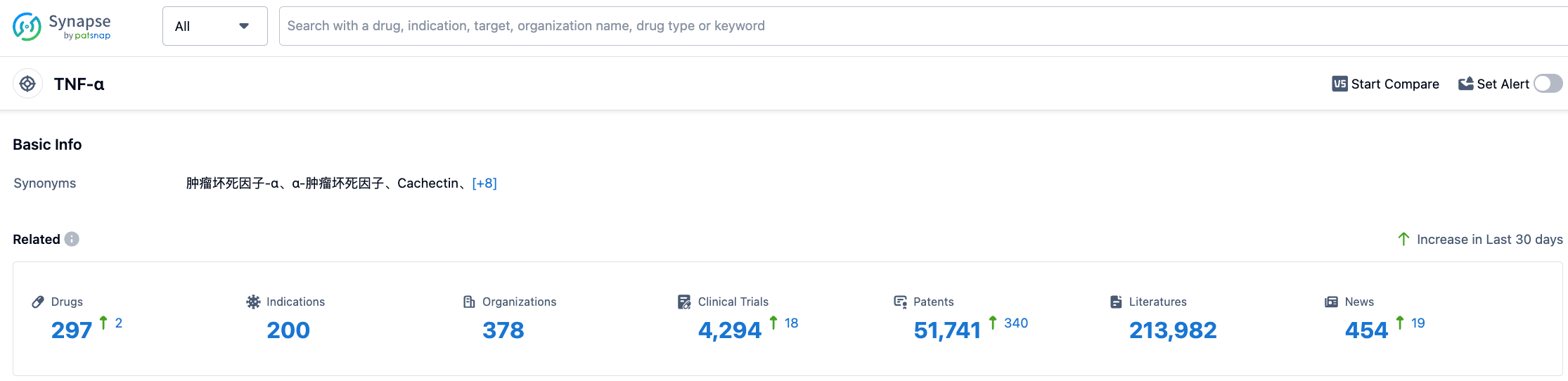
According to information disclosed by Synapse, as of August 2, 2023, there are 297 drugs under research targeting TNF-α, with 200 applicable diseases, 378 research institutions, 4293 related clinical trials, and as many as 51,763 patents. The research heat of TNF-α is quite high, mainly focusing on the field of autoimmune diseases. Its therapeutic effect on chronic inflammation is remarkable, and the industry looks forward to the subsequent performance of MYMD-1.